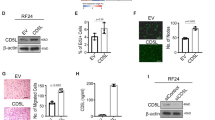
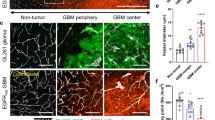
Abstract
Angiostatin is a naturally occurring inhibitor of angiogenesis that is being developed as a drug to fight cancer. In this study we reveal that EL-4 tumors established in mice rapidly develop resistance to angiostatin gene therapy by upregulating hypoxia-inducible pathways. Angiostatin initially delayed tumor growth for 6 days by reducing blood vessel density. However, tumors quickly responded by upregulating the production of hypoxia-inducible factor-1α (HIF-1α) and its effector vascular endothelial growth factor (VEGF) in response to increasing tumor hypoxia, leading to restored angiogenesis and rapid tumor growth. Theoretically, blockade of HIF-1 should prevent resistance to anti-angiogenic therapy by preventing a tumor from responding to induced hypoxia. Antisense HIF-1α inhibited the expression of HIF-1α and of the HIF-1 effectors VEGF, glucose transporter-1 and lactate dehydrogenase. As a monotherapy, it was effective in eradicating small 0.1 cm diameter tumors, but only delayed the growth of large 0.4 cm diameter tumors. In contrast, timed injection of a combination of angiostatin and antisense HIF-1α plasmids completely eradicated large EL-4 tumors within 2 weeks, and prevented upregulation of hypoxia-inducible pathways induced by angiostatin. The data indicate that blocking hypoxia-inducible pathways by antisense HIF-1α can circumvent hypoxia-induced drug resistance and thereby augment the efficacy of anti-angiogenic therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Folkman J . Tumour angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–1186.
Boehm T, Folkman J, Browder T, O'Reilly MS . Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997; 390: 404–407.
Cao Y . Antiangiogenic cancer therapy. Semin Cancer Biol 2004; 14: 139–145.
Garber K . Angiogenesis inhibitors suffer new setback. Nat Biotechnol 2002; 20: 1067–1068.
Casanovas O, Hicklin DJ, Bergers G, Hanahan D . Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumours. Cancer Cell 2005; 8: 299–309.
Drevs J, Zirrgiebel U, Schmidt-Gersbach CI, Mross K, Medinger M, Lee L et al. Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Ann Oncol 2005; 16: 558–565.
Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006; 24: 16–24.
Ding I, Sun JZ, Fenton B, Liu WM, Kimsely P, Okunieff P et al. Intratumoural administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res 2001; 61: 526–531.
Willett CG, Boucher Y, Duda DG, di Tomaso E, Munn LL, Tong RT et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol 2005; 23: 8136–8139.
Drevs J . Soluble markers for the detection of hypoxia under antiangiogenic treatment. Anticancer Res 2003; 23 (2A): 1159–1161.
Bocci G, Man S, Green SK, Francia G, Ebos JM, du Manoir JM et al. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res 2004; 64: 6616–6625.
Cao Y, Xue L . Angiostatin. Semin Thromb Hemost 2004; 30: 83–93.
O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79: 315–328.
O'Reilly MS, Holmgren L, Chen C, Folkman J . Angiostatin induces and sustains dormancy of human primary tumours in mice. Nat Med 1996; 2: 689–692.
Beerepoot LV, Witteveen EO, Groenewegen G, Fogler WE, Sim BK, Sidor C et al. Recombinant human angiostatin by twice-daily subcutaneous injection in advanced cancer: a pharmacokinetic and long-term safety study. Clin Cancer Res 2003; 9: 4025–4033.
Joseph JM, Bouquet C, Opolon P, Morizet J, Aubert G, Rössler J et al. High level of stabilized angiostatin mediated by adenovirus delivery does not impair the growth of human neuroblastoma xenografts. Cancer Gene Ther 2003; 10: 859–866.
Indraccolo S, Gola E, Rosato A, Minuzzo S, Habeler W, Tisato V et al. Differential effects of angiostatin, endostatin and interferon-alpha(1) gene transfer on in vivo growth of human breast cancer cells. Gene Therapy 2002; 9: 867–878.
Bussink J, Kaanders JH, van der Kogel AJ . Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol 2003; 67: 3–15.
Harris AL . Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47.
Fedele AO, Whitelaw ML, Peet DJ . Regulation of gene expression by the hypoxia-inducible factors. Mol Interv 2002; 2: 229–243.
Semenza GL . Life with oxygen. Science 2007; 318: 62–64.
Fulda S, Debatin KM . HIF-1-regulated glucose metabolism: a key to apoptosis resistance? Cell Cycle 2007; 6: 790–792.
Hockel M, Vaupel P . Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001; 93: 266–276.
Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW . Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Therapy 2001; 8: 638–645.
Liu F, Wang P, Jiang X, Tan G, Qiao H, Jiang H et al. Antisense hypoxia-inducible factor 1α gene therapy enhances the therapeutic efficacy of doxorubicin to combat hepatocellular carcinomas. Cancer Sci 2008; 99: 2055–2061.
Sun X, Jiang H, Jiang X, Tan H, Meng Q, Sun B et al. Downregulating hypoxia-inducible factor 1 augments transcatheter arterial embolization to treat hepatocellular carcinomas in rats. Hum Gene Ther 2009; 20: 314–324.
Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW . Angiostatin enhances B7.1-mediated cancer immunotherapy independently of effects on vascular endothelial growth factor expression. Cancer Gene Ther 2001; 8: 719–727.
Di Cristofano C, Minervini A, Menicagli M, Salinitri G, Bertacca G, Pefanis G et al. Nuclear expression of hypoxia-inducible factor-1alpha in clear cell renal cell carcinoma is involved in tumor progression. Am J Surg Pathol 2007; 31: 1875–1881.
Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 2007; 25: 911–920.
Mizukami Y, Kohgo Y, Chung DC . Hypoxia inducible factor-1 independent pathways in tumor angiogenesis. Clin Cancer Res 2007; 13: 5670–5674.
Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC . Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res 2004; 64: 1765–1772.
Abdollahi A, Lipson KE, Sckell A, Zieher H, Klenke F, Poerschke D et al. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res 2003; 63: 8890–8898.
Hinnen P, Eskens FA . Vascular disrupting agents in clinical development. Br J Cancer 2007; 96: 1159–1165.
Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE . HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J 2004; 23: 1949–1956.
Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res 2005; 65: 9047–9055.
Tan C, de Noronha RG, Roecker AJ, Pyrzynska B, Khwaja F, Zhang Z et al. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res 2005; 65: 605–612.
Acknowledgements
This work was supported by grants from the Wellcome Trust (UK), the World Health Organization, the Royal Society of New Zealand, the Cancer Society of New Zealand, the Health Research Council of New Zealand, the Lottery Grants Board of New Zealand, the Maurice and Phyllis Paykel Trust and the National Natural Scientific Foundation of China (30872987 and 30973474). GWK was supported by a James Cook Research Fellowship funded by the Royal Society of New Zealand. XS was supported by a Wellcome Trust Research Leave Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sun, X., Vale, M., Jiang, X. et al. Antisense HIF-1α prevents acquired tumor resistance to angiostatin gene therapy. Cancer Gene Ther 17, 532–540 (2010). https://doi.org/10.1038/cgt.2010.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2010.7
Keywords
This article is cited by
-
Cyclic glycine-proline regulates IGF-1 homeostasis by altering the binding of IGFBP-3 to IGF-1
Scientific Reports (2014)
-
Inhibition of the hypoxia-inducible factor pathway by a G-quadruplex binding small molecule
Scientific Reports (2013)
-
Gene expression and hypoxia in breast cancer
Genome Medicine (2011)
-
Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway
Leukemia (2011)