Abstract
Through silencing tumor suppressor genes, epigenetic changes can activate signaling pathways important to cancer development. In this report, we found an epigenetic contribution to the aberrant activation of wnt signaling in human gastric cancer. CXXC4 (CXXC finger protein 4) was identified as a novel target of EZH2 (enhancer of zeste homolog 2), and EZH2 promotes the activation of wnt singaling by downregulating CXXC4 expression. CXXC4 inhibits the growth of gastric cancer cells both in vitro and in vivo through inactivating wnt signaling. In contrast, depletion of CXXC4 activates wnt signaling and promotes the anchorage-independent growth of nontumor gastric epithelial cells. CXXC4 is downregulated in gastric carcinoma tissues and its downregulation is associated with poor outcome of gastric cancer patients (hazard ratio: 5.053, P<0.05). Through its binding to dishevelled (Dvl), CXXC4 stabilizes the destruction complex of β-catenin to inhibit wnt signaling. Two critical amino acid residues in CXXC4, K161 and T162 were found to be important to its binding to Dvl and the growth inhibitory effect of CXXC4. In summary, EZH2 promotes the activation of wnt signaling in gastric carcinogenesis through the downregulation of CXXC4 expression. CXXC4 is a novel potential tumor suppressor directly regulated by EZH2, and its expression is a significant prognosis factor for patients with early stages of gastric cancer.
Similar content being viewed by others
Main
As one of the most common malignancies, gastric cancer is the second leading cause of cancer death worldwide.1, 2 A great number of genetic and epigenetic alterations occur in gastric carcinogenesis, leading to the deregulation of many signaling pathways important to cell proliferation and differentiation.
Wnt/β-catenin signaling has key roles in embryonic development.3 In the absence of Wnt sigals, cytoplasmic β-catenin can be degraded by a destruction complex consisting of Axin, adenomatous polyposis coli (APC), casein kinase 1 (CK1) and glycogen synthase kinase-3 (GSK-3). CK1 and GSK-3 phosphorylate Ser/Thr residues in the N-terminus of β-catenin, which consequently facilitates β-catenin recognization by the E3 ubiquitin ligase β-Trcp, leading to its ubiquitination and proteasomal degradation. Once bound by Wnt ligands, the Frizzled (Fz)/low-density lipoprotein receptor-related protein 5/6 (LRP5/6) receptor complex activates the canonical Wnt signaling through it’s interaction with dishevelled (Dvl), which physically recruits Axin–GSK3, thus disrupting β-catenin destruction complex. As a result, β-catenin accumulates in the cytoplasm and translocates into the nucleus where it binds to T-cell factor/lymphoid enhancing factor and activates the transcription of Wnt target genes, such as cyclin D1 and c-myc.4, 5
Wnt signaling is also implicated in human diseases, including cancers.3, 6, 7, 8 For example, APC dysfunction could promote intestinal tumorigenesis both in human and animal models. Germline mutations in this gene cause familial adenomatous polyposis, an autosomal dominant pre-malignant disease that usually progresses to malignancy, mainly colorectal cancer. Aberrant activation of wnt signaling was frequently observed in other cancers, such as gastric cancer, although genetic changes of APC are relatively rare. It remains unclear how Wnt signaling is deregulated in the development of gastric cancer.
Recently, it has been reported that epigenetic changes contributes to the deregulation of wnt signaling in human cancers, such as hepatocellular carcinoma and breast cancer.9, 10 As the core catalytic subunit of PRC2 (Polycomb repressive complex 2) and highly conserved histone methyltransferase, enhancer of zeste homolog 2 (EZH2) represses gene transcription through trimethylating lysine-27 on histone H3 (H3K27me3).11 Overexpression of EZH2 is widely implicated in many malignancies.12, 13, 14 In the present study, we found CXXC4 (CXXC finger protein 4), a negative regulator of Wnt/β-catenin signaling, as a novel target of EZH2 in gastric cancer, and EZH2 promotes the activation of wnt singaling in gastric cancer through downregulating CXXC4 expression.
Results
Relevance of EZH2 to human gastric cancer
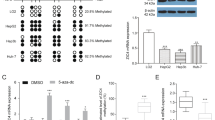
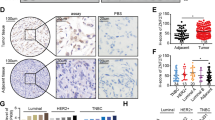
To clarify the relevance of EZH2 to human gastric carcinogenesis, EZH2 protein expression in human tissue samples, including primary gastric tumor tissues, gastritis and normal stomach tissues, was determined by immunohistochemical staining. EZH2 protein was overexpressed in gastric tumor tissues and also detectable in gastritis tissues, but was expressed at low level in normal tissues (Pearson’s χ2-test, P=0.004, Figures 1a and b). We further detected the expression of EZH2 in a panel of gastric cancer cell lines with western blotting. EZH2 was upregulated in most of the gastric cancer cell lines compared with its lower expression in GES-1, which is a nontumor human gastric epithelial cell line (Figure 1c). Functionally, ectopic expression of EZH2 promoted both the anchorage-dependent and -independent growth of GES-1 cells, as well as the expression of cyclin D1 and c-myc (Figures 1d and f). In contrast, cell growth and the expression of cyclin D1 and c-myc was inhibited after knockdown of EZH2 in gastric cancer cells (Figures 1g and h), suggesting that EZH2 contributes to the development of gastric cancer probably through the regulation of wnt signaling.
Relevance of EZH2 to human gastric cancer. (a and b) EZH2 expression in gastric tissues were determined by immunohistochemical staining (b), Pearson’s χ2-test, P=0.004). (c) The expression of EZH2 in gastric cancer cell lines and GES-1 cells were analyzed by western blotting. (d and e) The effect of ectopic EZH2 expression on the growth of GES-1 cells were determined by MTS assay (d) and soft agar assay (e). Results are shown as mean±S.D. Student’s t-test was used for the statistical analysis. The asterisk indicate significant statistical difference (P<0.05). (f) The effect of EZH2 overexpression on cyclin D1 and c-myc was investigated by real-time RT-PCR. The effect of EZH2 depletion on cell growth (g) and gene expression (h) was analyzed as in d and f, respectively
Identification of CXXC4 as a novel target of EZH2
EZH2 promotes carcinogenesis mainly through epigenetic silencing of tumor suppressor genes. Microarray analysis of gene expression before and after EZH2 depletion was performed to screen downstream targets of EZH2 in human gastric cancer cells. Among 44 841 reads, 419 genes were significantly upregulated after EZH2 depletion (Supplementary Table 2). As EZH2 regulates wnt signaling in gastric cancer, we focused on CXXC4, the negative regulator of Wnt/β-catenin signaling. It has been found to be regulated by EZH2 in human umbilical vein endothelial cells.15 We found that CXXC4 was significantly upregulated after knockdown of EZH2 in human gastric cancer cells (Figure 2a). In contrast, CXXC4 expression in GES-1 cells was dramatically inhibited after ectopic expression of EZH2 (Figure 2b), indicating that CXXC4 might be a novel target of EZH2 in stomach cells.
Identification of CXXC4 as a novel target of EZH2. CXXC4 expression before and after EZH2 depletion in MKN28 and SGC7901 cells (a), or ectopic EZH2 expression in GES-1 cells (b) were analyzed by real-time RT-PCR. (c) Expression of EZH2 and CXXC4 in primary tissues were determined by IHC and ISH, respectively. (d) The association of CXXC4 expression with EZH2 expression was analyzed by Pearson’s χ2-test (P<0.05). (e) The interaction of EZH2 with CXXC4 promoter region in MKN28 cells was analyzed by ChIP PCR
To further confirm the regulation of CXXC4 by EZH2, we examined the expression of EZH2 and CXXC4 in primary gastric tissues by immunohistochemical staining and in situ hybridization (ISH) assay, respectively. Consistent with results from in vitro cultured cells, CXXC4 was downregulated in tissues with high level of EZH2 expression, whereas it was highly expressed in tissues with weak EZH2 expression (Figures 2c and d).
To clarify whether CXXC4 is directly regulated by EZH2, we determined the physical interaction of EZH2 with CXXC4 promoter by chromatin immunoprecipitation (ChIP). EZH2 was indeed enriched on CXXC4 promoter region (Figure 2e), confirming that CXXC4 is a novel target directly regulated by EZH2.
Downregulation of CXXC4 in gastric cancer
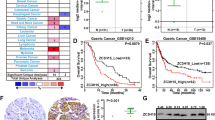
As EZH2 functions to promote cancer development through inhibiting the expression of tumor suppressors, we wonder whether CXXC4 is a novel tumor suppressor in gastric cancer. We first determined its expression in gastric cancer and found that CXXC4 expression is significantly downregulated in GC cell lines when compared with its expression in GES-1 cells (Figure 3a). Furthermore, CXXC4 expression was decreased in gastric tumor tissues compared with nontumor tissues (Figures 3b and c). To further clarify the clinical relevance of CXXC4 downregulation, we analyzed CXXC4 expression in 131 gastric cancer patients with survival data (Table 1). Interestingly, patients with positive CXXC4 expression seem to survive longer, although the statistical difference was not significant (Figure 3d). As staging is the most important factor to determine the clinical outcome of cancer patients, we further explored the influence of CXXC4 expression on the survival of patients with different stages. Kaplan–Meier survival curve revealed that CXXC4 expression status significantly influences the survival of patients with early stages of gastric cancer (Figure 3e). Both univariate and multivariate Cox-regression analyses confirmed that CXXC4 expression can influence the outcome of patients with early stages of gastric cancer (Figures 3f and g). Patients with reduced CXXC4 expression had a shorter survival.
Downregulation of CXXC4 in gastric cancer. (a) CXXC4 expression in GES-1 and gastric cancer cells were analyzed by real-time RT-PCR. (b and c) Expression of CXXC4 mRNA in gastric tumor tissues and nontumor tissues was tested by ISH (Pearson’s χ2-test, P<0.001). The association of CXXC4 expression status with survival of all gastric cancer patients (d) or patients with early stage of gastric cancer (stage I and II, e) was explored by the Kaplan–Meier survival analysis. Univariate (f) and multivariate (g) Cox regression analysis was used to confirm the prognosis value of CXXC4 expression. High expression is the reference. Diff. indicates differentiation with good differentiation as the reference. Lauren indicates Lauren types with intestinal type as the reference. TNM indicates TNM staging and stage I is the reference
CXXC4 functions as a tumor suppressor gene in gastric cancer
Next, we explored the potential tumor suppressor function of CXXC4 in gastric cancer. Overexpression of CXXC4 effectively reduced the growth of MKN28 and SGC7901 cells that have relatively low level of CXXC4 expression (Figure 4a). To further determine the long-term effect of CXXC4 on gastric cancer cells, we engineered SGC7901 cells to express CXXC4 in a doxycycline (Dox)-dependent manner (Figure 4b). The growth of SGC7901 cells in vitro was greatly inhibited in the presence of Dox (data not shown). In addition, Dox-induced CXXC4 expression also significantly reduced the tumorigenicity of SGC7901 in nude mice (Figures 5c and d). Moreover, knockdown of CXXC4 (Figure 4e) promoted the growth of GES-1 cells (Figure 4f). Importantly, the clonogenicity of GES-1 cells in soft agar was also enhanced (Figure 4g), strongly demonstrating the tumor suppressor function of CXXC4.
CXXC4 functions as a tumor suppressor in gastric cancer. (a) The effect of ectopic CXXC4 expression on gastric cancer cells was analyzed by MTS assay. The effect of CXXC4 expression on the growth of gastric cancer cells in vivo was analyzed by inoculating SGC7901 cells into nude mice. CXXC4 expressed only in the presence of Dox (b). The curve of tumor volumes and the weight of the tumor (Student’s t-test, P<0.05) were shown in c and d, respectively. The effect of CXXC4 depletion (e), determined by real-time RT-PCR) on the growth of GES-1 cells was determined by MTS assay (f) and soft agar assay (g)
CXXC4 inhibits wnt signaling in gastric cancer. (a) The level of β-catenin before and after CXXC4 expression were determined by western blotting. (b) The expression of wnt pathway target genes (cyclin D1 and c-myc) before and after CXXC4 expression were determined by real-time RT-PCR. (c) The effect of CXXC4 expression in the presence or absence of dominant active β-catenin on the growth of MKN-28 cells was explored by MTS assay. (d) The amount of β-catenin and phosphorylated GSK-3β in GES-1 cells before and after CXXC4 depletion were determined by western blotting. (e) The expression of cyclin D1 and c-myc before and after CXXC4 depletion were determined by real-time RT-PCR. (f) The effect of CXXC4 depletion in the presence or absence of β-catenin siRNA on the growth of GES-1 cells was explored by MTS assay. (g) The interaction of Dvl-1 with wild-type or mutated CXXC4 was explored by co-immunoprecipation assay. (h) The association of Dvl-1 with Axin 1, GSK-3 in the presence of wild-type or mutated CXXC4 was explored by co-immunoprecipation assay. (i) The amount of β-catenin or phosphorylated GSK-3β in the presence of wild-type or mutated CXXC4 were determined by western blotting
CXXC4 inhibits wnt signaling in gastric cancer
It has been reported that CXXC4 was a negative regulator of Wnt/β-catenin signaling in renal cell carcinoma (RCC).16 We wonder whether CXXC4 regulates wnt signaling in gastric cancer. Ectopic expression of CXXC4 indeed reduced intracellular level of β-catenin (Figure 5a) and the expression of two wnt targets, cyclin D1 and c-myc (Figure 5b). Furthermore, dominant positive β-catenin mutant refractory to proteasome-mediated degradation rescued the growth inhibitory effect of CXXC4 (Figure 5c), indicating that CXXC4 might inhibit gastric carcinogenesis through attenuating wnt signaling. In consistence with results of gain-of-function experiments, CXXC4 depletion increased the amount of β-catenin (Figure 5d) and the expression of cyclin D1 and c-myc (Figure 5e). Knockdown of CXXC4 failed to promote the growth of GES-1 cell once β-catenin was simultaneously depleted (Figure 5f), confirming the inhibitory effect of CXXC4 on wnt signaling and gastric cancer.
CXXC4 interacts with Dvl-1 directly and the mutation of critical residues in the Dvl-interacting domain abrogated its interaction with Dvl-1 (Figure 5g and Supplementary Figure 1).17 Importantly, the association of Dvl-1 with Axin 1 and GSK-3β was disrupted upon the expression of wild-type but not the mutated CXXC4 that failed to interact with Dvl-1 (Figure 5h). Moreover, the wild-type but not the mutated CXXC4 reduced the amount of β-catenin and inhibited the phosphorylation of GSK-3β (Figure 5i and Supplementary Figure 2). In contrast, knockdown of CXXC4 increased the phosphorylation of GSK-3β (Figure 5d and Supplementary Figure 2), indicating that CXXC4 functions to inhibit wnt signaling by stabilizing the destruction complex of β-catenin.
EZH2 activates wnt signaling through downregulating CXXC4
Next, we asked whether EZH2 activates wnt signaling to promote gastric carcinogenesis through inhibiting CXXC4 expression. Ectopic EZH2 expression increased the amount of β-catenin in GES-1 cells (Figure 6a), whereas EZH2 depletion reduced β-catenin expression in gastric cancer cells (Figure 6b). Growth inhibitory effect of EZH2 small interference RNA (siRNA) was reversed by dominant active β-catenin (Figure 6c), indicating that EZH2 activates wnt signaling to promote gastric carcinogenesis. EZH2-promoted cell growth and activation of wnt signaling were attenuated by ectopic CXXC4 expression (Figures 6d and e). Meanwhile, EZH2 siRNA failed to inhibit cell growth and β-catenin expression when CXXC4 expression was inhibited simultaneously (Figures 6f and g), indicating that EZH2 activates wnt signaling in gastric cancer mainly through the downregulation of CXXC4 expression.
EZH2 activates wnt signaling through downregulating CXXC4. The level of β-catenin before and after EZH2 overexpression (a) and depletion (b) was analyzed by western blotting. (c) The effect of EZH siRNA in the presence or absence of dominant active β-catenin mutant on the growth of MKN28 cells was determined by MTS assay. The effect of ectopic EZH expression in the presence or absence of CXXC4 on cell growth and β-catenin expression in GES-1 cells was determined by MTS assay (d) and western blotting (e), respectively. The effect of EZH siRNA in the presence or absence of CXXC4 siRNA on cell growth and β-catenin expression in MKN-28 cells was determined by MTS assay (f) and western blotting (g), respectively. (h) A proposed working model: The left panel indicates that CXXC4 stabilizes the destruction complex of β-catenin and reduces β-catenin expression, whereas the right panel shows that EZH2 can promote the downregulation of CXXC4 and thus prevents the degradation of β-catenin
Discussion
Aberrant activation of the canonical Wnt/β-catenin signaling induced by genetic changes, such as mutations in APC or β-catenin-endcoding CTNNB1, has been reported as one of driving forces in the progression of many cancers.18, 19 However, mutations in APC and CTNNB1 mutations are relatively infrequent in gastric cancer,20, 21 indicating that other mechanisms may be responsible for the activation of Wnt signaling in gastric carcinogenesis. Here we uncover that EZH2 promotes the activation of Wnt signaling through downregulating CXXC4 expression, representing an epigenetic mechanism of wnt signaling activation in gastric cancer cells (Figure 6h).
Our findings first illustrate that the epigenetic repression of CXXC4 by EZH2 results in the constitutive Wnt/β-catenin signaling activation in gastric cancer, which is consistent with the previous reports that EZH2 promoted carcinogenesis by repression of tumor suppressor genes, such as ADRB2, CDH1, PSP94 and DAB2IP.22, 23, 24, 25 These observations imply that epigenetic regulations, as an alternative to genetic changes, can also participate in the aberrant activation of Wnt/β-catenin signaling in cancer cells. Interestingly, promoter hypermethylation has been reported to contribute to the inactivation of some Wnt antagonists in human gastric cancer, such as secreted Fz-related proteins, Wnt inhibitory factor-1 and DICKKOPF family genes.26, 27, 28 Meanwhile, EZH2 target genes are often silenced by DNA methylation, as EZH2 is required for the recruitment of DNA methyltransferases (DNMTs) to facilitate CpG methylation.29 However, we found that DNA methylation is not involved in the downregulation of CXXC4 in human gastric cancer cells (data not shown). Recently, it has been reported that other negative Wnt regulators, such as DACT3 and RACK1, was transcriptionally repressed in human cancers independent of promoter methylation.30, 31 These observations suggest that histone modification has a significant role in the regulation of wnt signaling.
Dvl functions as a scaffold for Wnt/β-catenin signaling.32 When Wnt ligands bind to the Fz/LRP5/6 coreceptor complex to initiate the canonical Wnt cascade, Dvl–Axin association cooperatively induces downstream cascade activation by heterodimerization through their respective DIX domains.33
CXXC4 functions as a negative regulator of Wnt signaling in RCC.16, 34 However, how it can inhibit wnt signaling and how the regulation of its expression is in human carcinogenesis remain to be clarified. Here we provided evidence that overexpression of wild-type CXXC4 disrupts the association of Dvl with Axin–GSK-3β by directly interacting with Dvl, and consequently activates GSK-3β to promote the phosphorylation and degradation of β-catenin. Once the critical residues in the KTXXXI motif responsible for the interaction with Dvl1 are mutated, CXXC4 fails to interact with Dvl1 and loses its inhibitory effect on wnt signaling (Figure 5). Other Dvl-interacting proteins, such as DACT3 and Dapper1, have been reported to negatively regulate Wnt signaling in human cancer cells.30, 35 It would be interesting to explore whether EZH2 is relevant to regulate the expression of other Dvl-interacting proteins in human cancers.
Ectopic expression of CXXC4 in gastric cancer cell lines decreased cell growth both in vitro and in vivo, whereas depletion of CXXC4 promoted both anchorage-dependent and -independent cell growth, indicating that CXXC4 functions as a tumor suppressor in gastric cancer. Although dominant active β-catenin reversed CXXC4-induced growth inhibition and growth promoted by CXXC4 depletion was compromised once β-catenin was inhibited, we could not exclude other functions of CXXC4. For example, CXXC domain-containing proteins, such as DNMT1 (DNA methyltransferase 1), could interact with DNA. It remains unknown whether CXXC4 interacts with DNA through its CXXC domain. In addition, it has been hypothesized that the planar cell polarity pathway, including RhoA and Rac, might be activated by the downregulation of CXXC4.16 In addition, several reports have revealed that the C-terminal region of Idax shares homology with a putative MAPK and NF-κB activating protein (Q8TB79) containing the KTXXI motif that is necessary for Idax–-Dvl binding.36, 37 Recently, a Blast search confirmed that two leukemia-associated genes, LCX and MLL, containing the CXXC-zinc finger motif is homologous with xIdax, implying that Idax in human might be implicated in leukemogenesis.17
As the aberrant activation of wnt signaling occurs in many cancers, it is interesting to know whether the downregulation of CXXC4 is relevant to the development of other cancers, in addition to gastric cancer and RCC. Indeed, CXXC4 expression was found to be upregulated in human umbilical vein endothelial cells after EZH2 knockdown.15 The CXXC4 gene maps to 4q24 and the deletion of 4q has been observed in a wide variety of other cancers, including colorectal cancer, prostate cancer and hepatocellular carcinoma,38, 39, 40 indicating that CXXC4 downregulation caused by genetic changes might be associated with the development of human cancers. In addition, CXXC4 was also downregulated in tissues with lower EZH2 expression, indicating that CXXC4 can also be downregulated in an EZH2-independent manner.
In summary, CXXC4 is a direct target of EZH2 and is downregulated in human gastric cancer. CXXC4 functions as a tumor suppressor in gastric cancer and inhibits wnt signaling by competitively binding to the PDZ domain of Dvl and stabilizing β-catenin destruction complex to promote the degradation of β-catenin.
Materials and Methods
Cell lines and antibodies
Human gastric epithelial cell line GES-1 and gastric cancer cell lines were cultured in RPMI 1640 medium or DMEM (Life Technologies, Carsbad, CA, USA) supplemented with 10% fetal bovine serum as previously described.41 The immortalized but nontumorigenic GES-1 cell line was established from the fetal stomach cells infected with SV40 virus.42 Antibodies to EZH2, Myc-Tag, Phospho-GSK-3α/β (Ser21/9) and Axin1 were purchased from Cell Signaling Technology (Boston, MA, USA). Antibodies to β-catenin and CyclinD1 were obtained from Epitomics (Burlingame, CA, USA). Anti-Dvl-1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-trimethyl Histone H3 (Lys27) was obtained from Millipore (Billerica, MA, USA). Anti-c-myc antibody was purchased from BD Bioscience (Bedford, MA, USA).
RNA extraction and quantitative real-time RT-PCR
Reverse-transcription reaction was performed using 1 μg of total RNA with High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Gene expressions were determined by quantitative real-time PCR using SYBR Green Master Mix Kit and ABI 7500 Real-Time PCR System (Applied Biosystems). Human glyceraldehyde-3-phosphate dehydrogenase was used as an internal control of RNA integrity. Primers used are listed in Supplementary Table 1.
Clinical samples and immunohistochemsitry
Human gastric tissue samples, including primary gastric tumor tissues, gastritis and normal stomach tissues, were obtained from Sir Run Run Shaw Hospital, Zhejiang University, Zhejiang, China. Immunohistochemical staining was performed as previously described.41
ISH assay
ISH assay was performed using the CXXC4 mRNA ISH Assay Kit (BOSTER, Wuhan, China) according to the manufacturer’s instructions. Briefly, de-waxed and re-hydrated paraffin-embedded tissue sections were treated with 3%H2O2 for 5–10 min at room temperature and digested with pepsin in 3% citric acid at 37 °C for 5–10 min, and post-fixed with 1% paraformaldehyde. Sections were hybridized for 20 h at 42 °C with hybridization probe specific to CXXC4 and were incubated with biotinylated anti-digoxin antibody. To visualize the signal, the slides were incubated with 3,3′-diaminobenzidine tetrahydrochloride hydrate (Genentech, San Francisco, CA, USA) for 2–3 min. The sequence of CXXC4 probe is listed in Supplementary Table 1.
Plasmid construction and transfection
The open reading frame (ORF) of human CXXC4 was cloned into pCMV-3Tag-7 using BamHI and XhoI restriction sites. Two CXXC4 mutants (T162A and K161A) were constructed using QuikChange Site-Directed Mutagenesis Kit (Agilent, La Jolla, CA, USA). Primers used are listed in Supplementary Table 1. To generate the Dox-inducible CXXC4 expression vector, CXXC4 ORF was subcloned into pTRE-tight vector (Clontech Laboratories, Mountain View, CA, USA) using SacI and KpnI restriction sites. SGC7901 cells being transfected with pTet-On regulator (Clontech Laboratories) and pTRE-tight-CXXC4 plasmid were selected for 2 weeks with complete RPMI 1640 medium containing G418 (400 μg/ml) (AMRESCO, Solon, OH, USA).
To stably knock down CXXC4 expression, pSUPER-CXXC4 shRNA was constructed according to the protocols provided by the manufacturer (Oligoengine, Seattle, WA, USA). The sequence of oligos for insert preparation are listed in Supplementary Table 1. FuGENE HD (Roche Applied Science, Mannheim, Germany) were used for plasmid transfection.
siRNAs and transfection
siRNAs for EZH2, CXXC4 and β-catenin were synthesized by GenePharma (Shanghai, China).The sequences of siRNAs are listed in Supplementary Table 1. Cells were transfected with siRNA duplexes (10 nM) using Lipofectamine RNAiMAX transfection reagent (Life Technologies) according to the manufacturer’s instructions.
Soft agar assay
Cells were seeded in 0.5 ml of 0.33% (w/v) agar in RPMI 1640 medium containing 10% FBS overlaid on 0.5 ml RPMI 1640 medium containing 10% FBS of a 0.5% (w/v) bottom agar layer in 12-well plates. Plates were incubated at 37 °C for 2 weeks before colonies were photographed and counted. All of the experiments were performed in triplicate and repeated at least three times.
Western blotting analysis and antibodies
Cells were scraped and lysed in Cytobuster Protein Extraction Reagent (Novagen, Darmstadt, Germany) and protein concentrations were determined by Bio-Rad protein assay kit II (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of cellular protein were resolved by SDS-PAGE and transferred to PVDF membrane. Proteins of interest were detected as previously described.43
ChIP assay
ChIP assay was carried out using SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling Technology). In brief, 4 × 107 MKN28 cells were fixed with 1% formaldehyde. Antibodies specific to EZH2 (5 μg) or trimethyl Histone H3 (Lys27) (2 μg) were added and the complex was precipitated by Protein G magnetic beads. DNA recovered from the immunoprecipitated chromatin was subjected to PCR analysis. CXXC4 primers used for ChIP assay are listed in Supplementary Table 1.
Immunoprecipitation
Cells were lysed in 1 ml of lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 20 mg of leupeptin/ml, 20 mg of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride, 1% Nonidet P-40 and 10% glycerol). Primary antibodies (normally 1–2 μg) were incubated with the pre-cleared cell lysates overnight at 4 °C. The immunocomplexes were precipitated by incubating with 30 μl Pure Proteome Protein G magnetic beads.44
Cell growth assay
Cell proliferation assay was performed using CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay kit (Promega, Madison, WI, USA) and cell cycle distribution was determined by flow cytometry analysis as previously described.
Tumor growth in vivo
SGC7901 cells (1 × 107) with Dox-inducible CXXC4 expression vector were injected subcutaneously into the flank of nude mice. When tumors reached to 200 mm3, the mice were randomly divided into two groups by adding Dox (1 mg/ml) to the drinking water or not. Tumor volumes were measured by caliper measurements every 2 days and calculated according to the following formula: V=π/6 × f × (L × W)3/2, where V, volume (mm3); L, biggest diameter (mm); W, smallest diameter (mm), f(females)=1.58±0.01. The tumors were weighed at the end of the experiments.
Microarray analysis
The microarray analysis was performed in Shanghai KangChen Biotech (Shanghai, China) with NimbleGen gene profiling kits (Roche, Basel, Switzerland) (Array Type: Human_HX12_expr). Briefly, total RNAs extracted from MKN28 cells with or without EZH2 knockdown were amplified and labeled using a NimbleGen One-Color DNA Labeling Kit and hybridized in NimbleGen Hybridization System (Roche). Raw data were extracted as pair files by NimbleScan software (version 2.5). Differentially expressed genes were identified through fold-change screening and modified Student’s t-test of triplicate experiments.
Statistical analysis
The Student’s t-test was used to explore the difference in gene expression and cell growth. The Pearson’s χ2-tests were used to analyze the association of CXXC4 expression with EZH2 expression and clinical parameters. The probability of overall survival was calculated with the Kaplan–Meier method and differences between curves were evaluated with the log-rank test. Relative risks of death associated with CXXC4 expression and other predictor variables were estimated by the Cox proportional hazards model. All statistical analyses were performed using SPSS for Windows, version 14.0. A P-value <0.05 was taken as statistically significant.
Abbreviations
- EZH2:
-
enhancer of zeste homolog 2
- CXXC4:
-
CXXC finger protein 4
- siRNA:
-
small interference RNA
- Dvl:
-
dishevelled
References
Correa P . Helicobacter pylori infection and gastric cancer. Cancer Epidemiol Biomarkers Prev 2003; 12: 238s–241s.
Ushijima T, Sasako M . Focus on gastric cancer. Cancer Cell 2004; 5: 121–125.
Moon RT, Kohn AD, De Ferrari GV, Kaykas A . WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004; 5: 691–701.
He X, Semenov M, Tamai K, Zeng X . LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 2004; 131: 1663–1677.
MacDonald BT, Tamai K, He X . Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17: 9–26.
Polakis P . Wnt signaling and cancer. Genes Dev 2000; 14: 1837–1851.
Moon RT, Bowerman B, Boutros M, Perrimon N . The promise and perils of Wnt signaling through beta-catenin. Science 2002; 296: 1644–1646.
Logan CY, Nusse R . The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781–810.
Cheng AS, Lau SS, Chen Y, Kondo Y, Li MS, Feng H et al. EZH2-mediated concordant repression of Wnt antagonists promotes beta-catenin-dependent hepatocarcinogenesis. Cancer Res 2011; 71: 4028–4039.
Li X, Gonzalez ME, Toy K, Filzen T, Merajver SD, Kleer CG . Targeted overexpression of EZH2 in the mammary gland disrupts ductal morphogenesis and causes epithelial hyperplasia. Am J Pathol 2009; 175: 1246–1254.
Margueron R, Reinberg D . The Polycomb complex PRC2 and its mark in life. Nature 2011; 469: 343–349.
Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 2003; 100: 11606–11611.
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002; 419: 624–629.
Matsukawa Y, Semba S, Kato H, Ito A, Yanagihara K, Yokozaki H . Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci 2006; 97: 484–491.
Dreger H, Ludwig A, Weller A, Stangl V, Baumann G, Meiners S et al. Epigenetic regulation of cell adhesion and communication by enhancer of zeste homolog 2 in human endothelial cells. Hypertension 2012; 60: 1176–1183.
Kojima T, Shimazui T, Hinotsu S, Joraku A, Oikawa T, Kawai K et al. Decreased expression of CXXC4 promotes a malignant phenotype in renal cell carcinoma by activating Wnt signaling. Oncogene 2009; 28: 297–305.
Michiue T, Fukui A, Yukita A, Sakurai K, Danno H, Kikuchi A et al. XIdax, an inhibitor of the canonical Wnt pathway, is required for anterior neural structure formation in Xenopus. Dev Dyn 2004; 230: 79–90.
Barker N, Clevers H . Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov 2006; 5: 997–1014.
Li ZQ, Ding W, Sun SJ, Li J, Pan J, Zhao C et al. Cyr61/CCN1 is regulated by Wnt/beta-catenin signaling and plays an important role in the progression of hepatocellular carcinoma. PLoS One 2012; 7: e35754.
Nakatsuru S, Yanagisawa A, Ichii S, Tahara E, Kato Y, Nakamura Y et al. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet 1992; 1: 559–563.
Tong JH, To KF, Ng EK, Lau JY, Lee TL, Lo KW et al. Somatic beta-catenin mutation in gastric carcinoma--an infrequent event that is not specific for microsatellite instability. Cancer Lett 2001; 163: 125–130.
Chen H, Tu SW, Hsieh JT . Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J Biol Chem 2005; 280: 22437–22444.
Beke L, Nuytten M, Van Eynde A, Beullens M, Bollen M . The gene encoding the prostatic tumor suppressor PSP94 is a target for repression by the Polycomb group protein EZH2. Oncogene 2007; 26: 4590–4595.
Yu J, Cao Q, Mehra R, Laxman B, Tomlins SA, Creighton CJ et al. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell 2007; 12: 419–431.
Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 2008; 27: 7274–7284.
Guo Y, Guo W, Chen Z, Kuang G, Yang Z, Dong Z . Hypermethylation and aberrant expression of Wnt-antagonist family genes in gastric cardia adenocarcinoma. Neoplasma 2011; 58: 110–117.
Taniguchi H, Yamamoto H, Hirata T, Miyamoto N, Oki M, Nosho K et al. Frequent epigenetic inactivation of Wnt inhibitory factor-1 in human gastrointestinal cancers. Oncogene 2005; 24: 7946–7952.
Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S et al. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis 2007; 28: 2459–2466.
Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006; 439: 871–874.
Jiang X, Tan J, Li J, Kivimae S, Yang X, Zhuang L et al. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell 2008; 13: 529–541.
Deng YZ, Yao F, Li JJ, Mao ZF, Hu PT, Long LY et al. RACK1 suppresses gastric tumorigenesis by stabilizing the beta-catenin destruction complex. Gastroenterology 2012; 142: 812–823 e815.
Malbon CC, Wang HY . Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol 2006; 72: 153–166.
Clevers H . Wnt/beta-catenin signaling in development and disease. Cell 2006; 127: 469–480.
Hino S, Kishida S, Michiue T, Fukui A, Sakamoto I, Takada S et al. Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein. Mol Cell Biol 2001; 21: 330–342.
Zhang L, Gao X, Wen J, Ning Y, Chen YG . Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J Biol Chem 2006; 281: 8607–8612.
London TB, Lee HJ, Shao Y, Zheng J . Interaction between the internal motif KTXXXI of Idax and mDvl PDZ domain. Biochem Biophys Res Commun 2004; 322: 326–332.
Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y et al. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene 2003; 22: 3307–3318.
Brosens RP, Belt EJ, Haan JC, Buffart TE, Carvalho B, Grabsch H et al. Deletion of chromosome 4q predicts outcome in stage II colon cancer patients. Cell Oncol (Dordr) 2011; 34: 215–223.
Matsui S, LaDuca J, Rossi MR, Nowak NJ, Cowell JK . Molecular characterization of a consistent 4.5-megabase deletion at 4q28 in prostate cancer cells. Cancer Genet Cytogenet 2005; 159: 18–26.
Arai Y, Honda S, Haruta M, Kasai F, Fujiwara Y, Ohshima J et al. Genome-wide analysis of allelic imbalances reveals 4q deletions as a poor prognostic factor and MDM4 amplification at 1q32.1 in hepatoblastoma. Genes Chromosomes Cancer 2010; 49: 596–609.
Lam EK, Wang X, Shin VY, Zhang S, Morrison H, Sun J et al. A microRNA contribution to aberrant Ras activation in gastric cancer. Am J Trans Res 2011; 3: 209–218.
Ke Y, Ning T, Wang B . [Establishment and characterization of a SV40 transformed human fetal gastric epithelial cell line-GES-1]. Zhonghua zhong liu za zhi 1994; 16: 7–10.
Zhang S, Jiang T, Feng L, Sun J, Lu H, Wang Q et al. Yin Yang-1 suppresses differentiation of hepatocellular carcinoma cells through the downregulation of CCAAT/enhancer-binding protein alpha. J Mol Med (Berl) 2012; 90: 1069–1077.
Jin H, Sperka T, Herrlich P, Morrison H . Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature 2006; 442: 576–579.
Acknowledgements
This work was supported by the Qianjiang Scholar Program of Zhejiang province to XW (number 2011R10073) and Natural Science foundation of Zhejiang Province (number LR12H16001) to HJ.
Author Contributions
XW and HJ conceived and designed the experiments. HL, JS, FW, LF, YM, QS, ZJ and XS performed experiments. HL, XW and HJ analyzed data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Stephanou
Supplementary Information accompanies this paper on Cell Death and Disease website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Lu, H., Sun, J., Wang, F. et al. Enhancer of zeste homolog 2 activates wnt signaling through downregulating CXXC finger protein 4. Cell Death Dis 4, e776 (2013). https://doi.org/10.1038/cddis.2013.293
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.293
Keywords
This article is cited by
-
CXXC4 mediates glucose-induced β-cell proliferation
Acta Diabetologica (2020)
-
Genome wide association study identifies novel potential candidate genes for bovine milk cholesterol content
Scientific Reports (2018)
-
MiR-214 Regulates the Human Hair Follicle Stem Cell Proliferation and Differentiation by Targeting EZH2 and Wnt/β-Catenin Signaling Way In Vitro
Tissue Engineering and Regenerative Medicine (2018)
-
Prognostic significance of the methylation of Wnt pathway antagonists—CXXC4, DACT2, and the inhibitors of sonic hedgehog signaling—ZIC1, ZIC4, and HHIP in head and neck squamous cell carcinomas
Clinical Oral Investigations (2017)
-
The novel EZH2 inhibitor, GSK126, suppresses cell migration and angiogenesis via down-regulating VEGF-A
Cancer Chemotherapy and Pharmacology (2016)