Abstract
ΔNp63α, implicated as an oncogene, is upregulated by activated Akt, part of a well-known cell survival pathway. Inhibition of Akt activation by phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and the presence of putative p63-binding sites in the pten promoter led us to investigate whether ΔNp63α regulates PTEN expression. Knockdown of ΔNp63α led to increases in PTEN levels and loss of activated Akt, while overexpression of ΔNp63α decreased PTEN levels and elevated active Akt. The repression of PTEN by ΔNp63α occurs independently of p53 status, as loss of ΔNp63α increases PTEN expression in cell lines with and without functional p53. In addition, decreased levels of ΔNp63α resulted in an increase in nuclear PTEN. Conversely, in vivo nuclear PTEN was absent in the proliferative basal layer of the epidermis where ΔNp63α expression is highest. Additionally, we show that in keratinocytes a balance between ΔNp63α and PTEN regulates Akt activation and maintains normal proliferation rates. This balance is disrupted in non-melanoma skin cancers through increased ΔNp63α levels, and could enhance proliferation and subsequent neoplastic development. Our studies show that ΔNp63α negatively regulates PTEN, thereby providing a feedback loop between PTEN, Akt and ΔNp63α, which has an integral role in skin cancer development.
Similar content being viewed by others
Main
The p53 transcription factor family consists of the tumor suppressor p53 and the homologous p63 and p73. Unlike p53, p63 is essential for normal epidermal stratification and the proliferative potential of the epithelial stem cells.1, 2 p63 exists as various isoforms with contrasting functions.2 The TA isoforms (TAp63α, TAp63β and TAp63γ) have a full-length N-terminal transactivation domain, whereas the ΔN isoforms (ΔNp63α, ΔNp63β and ΔNp63γ) have a short but distinct transactivation domain. All isoforms have a DNA-binding domain that shares high homology with p53 that allows p63 proteins to bind to p53 DNA-binding sites.3, 4
Similar to p53, the TA isoforms of p63 and p73 can promote apoptosis and growth arrest through the induction of anti-proliferative genes. In contrast, the ΔN isoforms have been shown to induce pro-survival genes and inhibit anti-proliferative genes.4, 5, 6 Several studies indicate that ΔNp63α, the predominant isoform in adult tissue, may function as an oncogene as it can exert a dominant-negative effect over p53 and the TAp63 and TAp73 isoforms.2 Additionally, ΔNp63α is frequently overexpressed in a variety of squamous cell (SCC) and basal cell carcinomas (BCCs).7, 8
The survival factor Akt can increase ΔNp63α levels and in turn, ΔNp63α protects against UV-B-induced apoptosis via Akt activation.9, 10 However, the mechanism behind the positive feedback loop between ΔNp63α and Akt has not been described. Akt activation can be negated by phosphatase and tensin homolog deleted on chromosome 10 (PTEN). PTEN dephosphorylates phosphatidylinositol 3,4,5-trisphosphate, thereby inhibiting the signaling cascade that activates Akt. Additionally, PTEN can undergo nuclear localization to induce G1 arrest independently of Akt.11, 12, 13 The pten gene is induced by p53 and, considering that ΔNp63α can oppose p53 function and bind to p53 responsive elements (REs), it is likely that ΔNp63α may negatively regulate PTEN.2, 4, 14 In this study we report that loss of ΔNp63α results in upregulation of PTEN and subsequent decrease in Akt activation and cell proliferation. We demonstrated that a balance between ΔNp63α and PTEN expression is required to maintain the rate of normal proliferation, and disruption of this balance potentiates cell growth via activation of the Akt pathway. Finally, we showed that the ratio of ΔNp63α to PTEN expression was significantly perturbed in human non-melanoma skin cancers.
Results
ΔNp63α negatively regulates PTEN expression
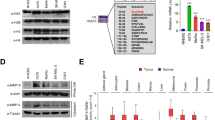
To address the potential functional interaction between endogenous p63 and PTEN, we first characterized the predominant p63 isoform in A431 and HaCaT. HaCaT cells were chosen because they are non-tumorigenic and represent an intermediary between normal and cancerous keratinocytes. A431 cells were derived from an atypical SCC of the vulva epidermis. Consistent with earlier reports, we demonstrated that ΔNp63α is the predominant isoform in both HaCaT and A431 cell lines.15, 16 A faint band corresponding to TAp63γ was detected upon immunoblot analysis using a TA-specific antibody (Supplementary Figure S1). To determine the effect of ΔNp63α on PTEN, we knocked down p63 in both mutant p53 (A431 and HaCaT, Figure 1a) and wild-type p53 (primary human keratinocytes, Figure 1b) backgrounds. Silencing p63 with multiple pan-p63 siRNAs led to increased PTEN levels independently of p53 status (Figures 1a and b). Increased IGFBP-3 levels, known to be negatively regulated by ΔNp63α, served as a positive control.5 We confirmed that the pan-p63 siRNAs led to knockdown of both TAp63 and ΔNp63 by isoform-specific qRT-PCR (Supplementary Figure S1C). Loss of only ΔNp63α by a ΔNp63-specific siRNA still increased PTEN expression, confirming that ΔNp63α is the main regulator of PTEN (Figure 1c). Although the increase in PTEN protein seems modest, minute changes in PTEN protein levels have profound biological impact and thus the modest increase we observe is significant.17 PTEN levels were also elevated in E1A-transformed murine embryonic fibroblasts (MEFs) and primary MEFs obtained from p63−/− mice when compared with wild-type mice (Figure 1d). A minor amount of residual p63 mRNA was detectable in the p63−/− MEFs because of the location of primers used for qRT-PCR, which produces a non-functional protein.18 Taken together, the results from these sets of experiments indicate that PTEN is negatively regulated by ΔNp63α.
ΔNp63α negatively regulates PTEN expression. (a) A431, HaCaT and (b) HEK cells were transfected with non-silencing control (NSC) siRNA or two to three different siRNAs targeting p63. Total RNA was extracted and transcript levels of p63, PTEN and IGFBP-3 were analyzed by qRT-PCR. Y-axis represents the fold change in PTEN and p63 transcript levels relative to NSC-transfected cells. Immunoblots of p63 and PTEN in A431 and HaCaT cells transfected as in (a) and HEKs as in (b) are shown in the bottom panels. Fold change in protein relative to NSC is listed above each band. (c) HaCaT cells were transfected with NSC or siRNA specific to ΔNp63 isoforms of p63. Total RNA and protein were extracted and analyzed as in (a). (d) Total RNA extracted from both primary and E1A-transformed MEFs obtained from wild-type and p63−/− mice were subjected to qRT-PCR to detect transcript levels in the top panel. The bottom panel represents immunoblot analysis of whole-cell extracts from wild-type and p63−/− MEFs. Note, p63 was undetectable at the protein level and near the limit of detection for qRT-PCR in p63−/− MEFs. Error bars represent standard deviation. *P<0.05 compared with control cells in all panels
ΔNp63α promotes survival of squamous carcinomas of the head and neck by repressing p73-dependent pro-apoptotic effects.19 The levels of both TA and ΔNp73 (Supplementary Figure S2) were examined, and ΔNp73 was the predominant isoform observed, suggesting that these cells may have a deficient p73-dependent pro-apoptotic program (Supplementary Figure S2). However, low levels of TAp73α and β were still detectable. To rule out the possibility that silencing ΔNp63α was inducing PTEN expression by reversing its dominant-negative effects on TAp73 or TAp63, we transfected cells with siRNA to ΔNp63α and/or siRNA specific to either TAp63 or TAp73 (Supplementary Figure S3). Importantly, we did not observe a reduction in PTEN expression upon knockdown of either the TAp63 or the TAp73 isoforms, ruling out the possibility that the TA isoforms in these cells were positively transcriptionally regulating PTEN (Supplementary Figure S3). Dual knockdown of ΔNp63α and TAp63 or ΔNp63 and TAp73 led to significant PTEN inductions similar to silencing ΔNp63 alone, concluding that PTEN induction observed upon silencing ΔNp63α occurs independently of any effects on TAp63 or TAp73 activity.
PTEN is a direct target of ΔNp63α
Examination of the pten promoter revealed the presence of several putative p63 REs in addition to the p53 RE described previously (Figure 2a).14 To define the region within the pten promoter to which ΔNp63α binds, we carried out chromatin immunoprecipitation (ChIP) assays in A431 and HaCaT cells using antibodies that recognize all p63 isoforms or only the p63α isoforms. p63 bound to the same locations of the pten promoter with similar affinities in both cell lines (Figure 2b). Similar to other genes, p63 also bound the p53 RE (region C, Figure 2b).4 Region D contained two p63-specific REs, yet failed to demonstrate binding. Region E only showed binding when immunoprecipitated with the pan-p63 antibody, indicating weak binding to this RE. Because mutant p53 has been demonstrated to complex with p63 and affect its binding to transcriptional targets, we also evaluated the binding of p53 to the pten promoter.20, 21 p53 bound nearly all of the same regions of the pten promoter as p63 (Supplementary Figure S4). Consistent with previous reports, the mutant p53 in A431 cells failed to bind the p21 promoter, suggesting that this mutation disrupts the normal binding pattern of p53.22 Contrary to previous studies, our results suggest that although mutant p53 may interact with p63, it does not prevent p63 from binding to the promoter of pten.20, 21 These results show that endogenous ΔNp63α is capable of binding to the pten promoter in multiple locations unique to p63 in addition to binding the p53 RE.
PTEN is a direct target of ΔNp63α. (a) Schematic of putative p63-binding sites and documented p53-binding sites in the pten promoter (accession no. AF067844). (b) ChIP assay was performed on A431 and HaCaT cells, wherein chromatin was immunoprecipitated with normal IgG control antibody or antibodies that recognize all p63 isoforms or p63α isoforms as indicated. Eluted DNA was PCR-amplified with primers specific for multiple regions of the pten promoter as shown in (a). The p21 promoter was used as a positive control for p63 binding. (c) The top left panel shows dual luciferase assays preformed on H1299 cells co-transfected with the full-length PTEN-Luc reporter and empty vector or ΔNp63α. The top right panel shows dual luciferase assays performed on HaCaT cells after knockdown of p63 by siRNA. Y-axis represents fold change in relative luciferase units compared with control-transfected cells. Representative immunoblots are shown below as proof of adequate overexpression or knockdown. The bottom panel shows dual luciferase assays in H1299 after co-transfection of the designated minimal PTEN-luc reporter and empty vector or ΔNp63α. The sequence corresponding to region B is shown below, with the core sequence of the putative p63 REs bolded and underlined. Lined up underneath each p63 half site is the canonical p63 RE, where R denotes purines; W, A or T; and Y, pyrimidines. Error bars represent standard deviation. *P<0.05 compared with control cells
To define the minimal region within the pten promoter critical for ΔNp63α-mediated repression of PTEN, we analyzed the effect of ΔNp63α on a luciferase reporter containing the full-length pten promoter (PTEN-Luc). p63 and p53 null H1299 cells transfected with ΔNp63α led to a statistically significant reduction of PTEN-Luc reporter activity when compared with control cells (Figure 2c, top panel). Silencing ΔNp63α in HaCaT cells led to a modest but reproducible increase in the PTEN-Luc reporter activity (Figure 2c). We next generated minimal luciferase reporters containing only the putative p63 REs found in the regions within the pten promoter that showed p63 binding by ChIP (Figure 2b). Co-transfecton of these reporters with ΔNp63α exhibited a significant reduction in reporter activity only with the minimal reporter corresponding to region B (Figure 2c, lower panel). These results suggest that negative regulation of PTEN by ΔNp63α is likely due to binding of ΔNp63α to region B of the pten promoter.
A balance between ΔNp63α and PTEN controls cell proliferation
To examine whether ΔNp63α promotes cell proliferation by inhibiting PTEN, colony-formation assays were carried out after silencing ΔNp63α and/or PTEN. Silencing ΔNp63α reduced colony formation, whereas silencing PTEN led to an increase in total colony number and in colony size. Interestingly, no statistical difference in colony number was observed when both ΔNp63α and PTEN were silenced when compared with control-transfected cells (Figure 3a). Silencing of ΔNp63α and PTEN was confirmed by immunoblot analysis as shown in Figure 3a. Consistent with this, cell proliferation determined by MTS assay, which measures the amount of actively metabolizing cells (Figure 3b), or cell viability determined by trypan blue exclusion (Figure 3c) also demonstrated that silencing both ΔNp63α and PTEN resulted in cell growth rates comparable to control cells. These results, based on three independent measures of cell growth, suggest that a critical balance between PTEN and ΔNp63α must be reached to maintain normal proliferation as perturbation of either PTEN or ΔNp63α levels affects proliferation.
A balance between ΔNp63α and PTEN controls cell proliferation. (a) A431 and HaCaT cells were transfected with NSC siRNA, p63 siRNA and/or PTEN siRNA as indicated. The left panel shows representative images of crystal violet-stained colonies at 3 days post re-seeding of cells transfected with designated siRNA or NSC. y-axis represents total number of colonies from (a) as counted with ImagePro 6.2. Error bars represent standard deviation. *P<0.05. Adequate knockdown was confirmed by immunoblot analysis and a representative experiment is shown. (b and c) A431 and HaCaT cells were transfected with NSC siRNA, p63 siRNA and/or PTEN siRNA as indicated and replated 24 h post transfection. Cells were subjected to MTS assays (b) or trypan blue staining (c) on the indicated day. y-axis represents total cell number (c). Error bars representing standard deviation within a single representative experiment
Activation of the Akt pathway via ΔNp63α-mediated PTEN repression
We next examined whether ΔNp63α-mediated regulation of PTEN affects Akt activation. Increasing levels of ΔNp63α led to a significant, dose-dependent reduction in endogenous PTEN protein levels with a concomitant increase in phosphorylated Akt (p-Akt) levels (Figure 4a). Induction of p-Akt by ΔNp63α is dependent on PTEN as overexpression of ΔNp63α in PTEN null PC3 cells failed to increase p-Akt levels (Figure 4b). We next wanted to determine whether ΔNp63α and constitutively active Akt (CA-Akt) had a synergistic effect on reduction of PTEN levels.10 ΔNp63α alone led to a dramatic reduction in PTEN expression, while CA-Akt alone led to a modest decrease in PTEN (Figure 4c). Co-expression of ΔNp63α and CA-Akt led to an enhancement of ΔNp63α, and PTEN was further reduced as compared with CA-Akt alone (Figure 4c). The ability of ΔNp63α alone to reduce PTEN expression suggests that ΔNp63α amplification is comparable to mutations in the PI3K/Akt pathway with regard to deregulating tumor suppression. Silencing ΔNp63α led to an increase in endogenous PTEN corresponding to decreased p-Akt levels (Figure 4d). Consistent with the return to normal growth rates when both ΔNp63α and PTEN were silenced in Figure 3, loss of both ΔNp63α and PTEN results in p-Akt levels similar to those of control-treated cells (Figure 4e). These data demonstrate that ΔNp63α and PTEN work in concert to modulate p-Akt levels to control proliferation rates.
ΔNp63α affects Akt activation by regulating PTEN expression. (a) H1299 cells were transfected with increasing concentrations (0–3 μg) of ΔNp63α as indicated. (b) PTEN null PC3 cells were transfected with empty vector or ΔNp63α and subjected to immunoblot analysis for the indicated proteins. (c) H1299 cells were transfected with ΔNp63α alone or together with constitutively active Akt (CA-Akt), and whole-cell lysates were subjected to immunoblot analysis as indicated. (d) HaCaT cells were transfected with three different siRNAs targeting p63 or NSC as indicated, and whole-cell extracts were subjected to immunoblot analysis as indicated. (e) HaCaT cells were treated with NSC or siRNA against p63 and/or PTEN, and whole-cell lysates were subjected to immunoblot analysis as indicated. Fold change in protein level, normalized β-actin or to Total Akt and β-actin, is listed above each band
ΔNp63α suppresses nuclear PTEN
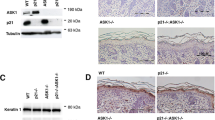
Although cytoplasmic PTEN antagonizes the Akt pathway, nuclear PTEN is associated with cell-cycle arrest that may be independent of Akt.13, 23 Therefore, we examined whether silencing ΔNp63α affects both nuclear and cytoplasmic PTEN levels. A significant increase in nuclear PTEN was observed when ΔNp63α was silenced (Figure 5a). Similarly, silencing ΔNp63α also led to increased nuclear PTEN at a cellular level, and non-silenced cells showed little nuclear PTEN (Supplementary Figure S5). Although there were increases in nuclear and cytoplasmic PTEN levels with a corresponding decrease in p-Akt levels, only the decrease in cytoplasmic p-Akt was statistically significant, supporting the hypothesis that nuclear PTEN has some functions that are independent of Akt (Figure 5a). ΔNp63α is often amplified in SCCs, so we next explored the effect of ΔNp63α overexpression on nuclear PTEN. As seen in Figure 5b, ectopic ΔNp63α led to a marked reduction of cytoplasmic PTEN with a corresponding increase in cytoplasmic p-Akt levels. A small, but reproducible, reduction in nuclear PTEN was also observed that led to a robust increase in nuclear p-Akt.
ΔNp63α suppresses nuclear PTEN. (a) HaCaT cells were transfected with NSC or p63 specific siRNA and subjected to subcellular fractionation. Immunoblot analysis of the indicated proteins is shown in the left panel. Nuclear protein levels were normalized to the intensity of PARP, while α-tubulin served as a loading control for normalization of cytoplasmic proteins. Fold change in protein level is listed above each band. (b) H1299 cells were transfected with empty vector or ΔNp63α before subcellular fraction and immunoblot analysis as in (a). (c) Indirect immunofluorescence was performed on normal adult human skin and E18.5 mouse skin. Representative images for nuclei (DAPI), p63 and PTEN are shown for each skin type. Basal layer cells that lack nuclear PTEN are shown by arrows, while an asterisk shows supra-basal layer cells lacking nuclear PTEN
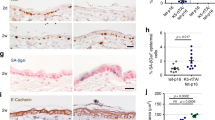
As nuclear PTEN is abundant in differentiated cells while ΔNp63α is associated with proliferating cells, we monitored the localization of PTEN and ΔNp63α in normal adult human and embryonic mouse epidermal tissues.11 Keratinocytes of the basal layer and hair follicles, with high p63 expression, showed nearly complete nuclear exclusion of PTEN (Figure 5c). PTEN staining was only observed in the nucleus of supra-basal layer cells that no longer expressed ΔNp63α (Figure 5c). ΔNp63α expression is normally downregulated as keratinocytes differentiate; however, in keratinocytes of the supra-basal layer that have yet to degrade ΔNp63α, no nuclear PTEN is observed (Figure 5c, marked by asterisk). The localization patterns of ΔNp63α and PTEN in intact normal human and mice skin, as well as in transformed keratinocyte cell lines, suggest that ΔNp63α inhibits localization of PTEN to the nucleus.
ΔNp63α and PTEN localization and expression in non-melanoma skin cancers
Elevated levels of ΔNp63α have been reported in a variety of SCC and BCC.15, 24, 25 On the basis of the observations in Figure 5 that overexpression of ΔNp63α could repress nuclear PTEN, we next sought to determine whether the amplification of ΔNp63α seen in human non-melanoma cancers coincided with reduced nuclear PTEN. The expression and localization of p63 and PTEN was examined in normal skin cores (n=9), BCC cores (n=11) and SCC cores (n=11) using a tissue microarray (Figure 6). In normal tissue, PTEN staining was most abundant in the cytoplasm of epidermal keratinocytes (Figure 6, top left panel). A few cells with intense PTEN staining were seen in the dermis and in melanocytes, consistent with earlier reports.26 It is important to remember that these cells are of mesenchymal origin and do not express any p63. In both BCC and SCC, very little nuclear PTEN was observed in the cancerous tissue. Intense staining for PTEN within the dermis was observed in areas of solar elastosis, partially due to some non-specific uptake of secondary antibodies (Figure 6, identified as SE in the bottom panel). The levels of p63 staining in BCC and SCC were considerably higher than in normal tissue, with all cancerous islands staining positive for p63. On the basis of the results shown in Figure 5, the high levels of p63 seen in Figure 6 could possibly be contributing to the lack of nuclear PTEN in BCC and SCC.
Elevated levels of ΔNp63α and reduced nuclear PTEN expression in basal and SCC. Indirect immunofluorescence was performed on a tissue microarray containing multiple cores of (a) normal human skin, (b) BCC and (c) SCC. Representative images of nuclei (DAPI), p63 and PTEN staining within each type of skin are shown. Areas of increased magnification to show PTEN localization are shown surrounded by a box. The regions within the tissue types are defined in the bottom panel. E, normal epidermis; D, dermis; SE, solar elastosis; BCC, basal cell carcinoma; SCC, squamous cell carcinoma
We next sought to determine whether there was any correlation between p63 and PTEN expression in the healthy and cancerous skin samples. An increase in p63 staining, as measured by the mean fluorescence intensity (MFI), was observed in BCC and SCC when compared with normal epidermal tissue (Figure 7a). A significant correlation was observed between the MFI values of p63 and PTEN in normal tissue (r=0.693, P=0.042), and in BCC (r=0.6687, P=0.00268) and SCC (r=0.7356, P=0.012). Although there was no overall decrease in PTEN staining accompanying the increase in p63 in cancerous samples, there was a major perturbation in the ratio of p63 to PTEN. A statistically significant difference (ANOVA F2, 29=12.915, P<0.0005) in the ratio between p63 and PTEN MFIs was observed when normal tissue was compared with BCC and SCC (Figure 7b), highlighting the importance of the balance between ΔNp63α and PTEN in controlling proliferation in the context of skin cancer.
Elevated levels of ΔNp63α in basal and SCC correlate with PTEN expression. (a) Quantification of the MFI for p63 and PTEN staining, in arbitrary units, from normal epidermal tissue, BCC and SCC. Error bars represent standard error of mean. (b) The average ratio of p63 fluorescence intensity to PTEN fluorescence intensity is higher in BCC and SCC as compared with normal skin samples. Error bars represent standard error of mean. *P<0.0005
Discussion
p63 is critical for epidermal stratification and the proliferative potential of the epithelial stem cells, but is also frequently overexpressed in SCCs.1, 2, 15, 24, 25 Skin-specific knockout of PTEN in mice leads to hyperproliferative and thickened skin.27 The opposing phenotypes of PTEN and p63 in epidermal regulation suggested that these two proteins may integrate into a common pathway. ΔNp63α controls the rate of proliferation in epithelial basal cells, thereby maintaining a crucial number of basal stem cells, whereas deletion of PTEN in the basal cells leads to uncontrolled cell proliferation.1, 28 This proliferation is considered to be an important event for initiation and progression of prostate cancer and may occur in other cancers, such as non-melanoma skin cancers.28 Here we have demonstrated for the first time that ΔNp63α negatively regulates PTEN expression, and subsequently enhances Akt activation, creating a positive feedback loop that regulates keratinocyte proliferation, and perturbation of this axis might lead to epithelial cancers.
It has been shown earlier that head and neck SCCs are reliant on high levels of ΔNp63α for survival through repression of p73-mediated apoptosis.19 Our studies, however, show that in at least epidermal cells TAp73 has little role in the transcriptional regulation of PTEN in the absence of genotoxic stress (Supplementary Figure S3). Previous studies demonstrated that TAp73 can induce PTEN transcription, suggesting that regulation of PTEN by the different p53 family members may be tissue specific.29 Furthermore, in our studies we failed to detect p63 binding to the same region as p73 (region D), suggesting that p63 and p73 control PTEN expression through different REs.29 Both TAp73 and TAp63 do not seem to induce PTEN expression, suggesting that ΔNp63α-mediated PTEN repression is independent of TAp63 and TAp73 (Supplementary Figure S3).
Although p53 is capable of inducing the transcription of PTEN, ΔNp63α functions as a master regulator independently of p53 to repress PTEN, as increases in PTEN expression were observed in A431 and HaCaT cells, which harbor different p53 mutations (R273H and p53Y179/W282, respectively), and in primary human epidermal keratinocytes (HEKs) with wild-type p53. Our results wherein we observed that p53 bound to many of the same regions within the pten promoter where p63 binds (Supplementary Figure S4) could be explained by a previous report that has shown that mutant p53 can bind to p63.20 Luciferase assays carried out in the absence of any p53 in cells overexpressing ΔNp63α still show repression of PTEN-Luc activity (Figure 2c), suggesting that although it is possible for mutant p53 to bind ΔNp63α, it does not alter the effects of p63 on PTEN regulation. It would be interesting to examine whether the differences in p53 mutational status affected other p63 target genes or whether the different p53 mutations dictate the tumorigenic and non-tumorigenic natures of A431 and HaCaT cells, respectively.
Mutations in the PI3K/Akt pathway are common in many types of cancers and contribute to the highly proliferative nature of tumors. Although overexpression of both ΔNp63α and CA-Akt did not lead to a synergistic or additive decrease in PTEN expression, it did result in a larger decrease in PTEN expression compared with CA-Akt alone (Figure 4b). ΔNp63α is more capable of repressing PTEN expression than CA-Akt alone, possibly due to the ability of active Akt to initiate the ARF/Egr-1 axis that induces PTEN to serve as a natural negative feedback mechanism.30 The potent ability of ΔNp63α to reduce PTEN expression suggests that ΔNp63α amplification may be enough to tip the balance toward hyperplasia and cancer development. ΔNp63α may also be capable of influencing proliferation by controlling nuclear levels of PTEN. Nuclear PTEN can elicit cell-cycle arrest independently of Akt, in part by binding to the anaphase-promoting complex to enhance cell-cycle repression.13, 31 Our results in Figure 5 show that modulation of ΔNp63α predominantly affects nuclear PTEN, supporting the hypothesis that ΔNp63α can control proliferation in both an Akt-dependent and -independent manner by repressing pten gene expression and by regulating PTEN localization, respectively. It remains to be seen, however, how ΔNp63α is capable of regulating the nuclear import and/or export of PTEN. Given that little nuclear PTEN is observed in basal layer keratinocytes of normal skin, it is unlikely that the lack of nuclear PTEN seen in BCC and SCC could be used as a prognostic marker (Figure 6). Instead, the dramatic lack of nuclear PTEN in both BCC and SCC, presumably due in part to amplification of ΔNp63α, might prevent cells from undergoing a proper cell-cycle arrest program.
As minute reductions in PTEN are sufficient to drive tumorigenesis, it is possible that changes in proteins that regulate PTEN, such as ΔNp63α, could also contribute to tumor formation.17 We observed differences in the magnitude of proliferation after silencing PTEN or ΔNp63α between cell lines (Figure 4b). A431 cells were less susceptible to changes in growth rates, possibly due to the atypical nature of this SCC line. However, both A431 and HaCaT cells showed similar trends in that silencing both ΔNp63α and PTEN resulted in nearly normal growth rates, suggesting that both cell lines utilize the ΔNp63α/PTEN axis to coordinate cell proliferation. The importance of the balance between ΔNp63α and PTEN is further highlighted in SCC and BCC, the two most common cancers, where the ratio of p63 to PTEN is significantly perturbed (Figure 6). BCCs often display wild-type p53 and rarely invade into the surrounding tissue, whereas SCCs usually have mutated p53 and a higher propensity for invasion.32, 33 The amplification of ΔNp63α and the subsequent imbalance between ΔNp63α and PTEN in both BCC and SCC suggests that regulation of PTEN in keratinocytes relies primarily on ΔNp63α to regulate proliferation, and disruption of the ΔNp63α/PTEN axis is an early insult contributing to both forms of skin cancer. Together, this study demonstrates that ΔNp63α maintains normal cell proliferation by negatively regulating basal PTEN levels and that disruption of this balance could potentiate cell growth via activation of the Akt pathway, as well as through repression of nuclear PTEN.
Materials and Methods
Cell lines and transfections
H1299, A431, HaCaT, PC3 and E1A-transformed primary mouse embryonic fibroblasts (gifted by Dr. Elsa Flores, MD Anderson) were maintained as described earlier.34 Primary human epidermal keratinocytes were maintained in Epilife media supplemented with human keratinocyte growth supplement and 100 U/ml penicillin and streptomycin and 0.25 μg/ml amphotericin B (Cascade Biologics, Portland, OR, USA). The ΔNp63α and CA-Akt plasmids used were described previously.34, 35 H1299 cells were transfected using Lipofectamine 2000, and A431, HaCaT and HEK cells were transfected with two rounds of siRNA using Oligofectamine as per the manufacturer's instructions (Invitrogen, Carlsband, CA, USA). Cells were harvested for immunoblot and qRT-PCR studies 24 h after the second transfection. Three different siRNAs (sip63-1, sip63-2 and sip63-3) were used to silence p63 to rule out off-target effects. Sequences used for silencing total p63, TAp63 and ΔNp63 were described previously.34, 36, 37 Sequences for silencing TAp73 were described previously.38 Sequences used for silencing PTEN are sense r(5′-GGCGUAUACAGGAACAAUA-3′)dTdT and antisense r(5′-UAUUGUUCCUGUAUACGCC-3′)dTdT.
RNA isolation and TaqMan real-time PCR studies
Quantitative real-time PCR analysis was carried out as previously described using Assay on Demand (AOD) specific for genes of interest and normalized to endogenous GAPDH for human genes or to β-actin for murine genes of interest (PE Applied Biosystems, Foster City, CA, USA).34 Human AODs used were GAPDH (4325792), p63 (Hs_00978340_m1), ΔNp63 (Hs_00978339_m1), TAp63 (Hs_00186613_m1), TAp73 (Hs_01056228_m1), ΔNp73 (Hs_1065727) IGFBP-3 (Hs_00426287_m1) and PTEN (Hs_00829813_s1). Murine AODs were p63 (Mm00495788_m1), PTEN (Mm00477210_m1) and β-actin (Mm00607939_s1).
PTEN transactivation assays
The full-length PTEN-luc reporter used was described previously.39 Cells were transfected with 100 ng of reporters containing the full-length PTEN promoter or 500 ng of the designated minimal PTEN promoter reporter along with the ΔNp63α or empty vector and Renilla luciferase expression vector to control for transfection efficiency. Dual-luciferase assays were carried out using Dual-Luciferase Reporter 1000 Assay System as per manufacturer's protocol (Promega, Madison, WI, USA). The minimal PTEN luciferase reporters were created by annealing primers to the regions of interest followed by ligation into the pGL3 Basic promoter-less luciferase reporter (Promega) at the KPNI and HINDIII sites. The primers used were: A forward 5′-CAAACGAGCCGAGTTACCGGGGAAGCGAGAGGTGGGGCGCTGCAAGGGAA-3′, A reverse 5′-AGCTTTCCCTTGCAGCGCCCCACCTCTCGCTTCCCCGGTAACTCGGCTCGTTTGGTAC-3′; B forward 5′-CCAGCGTGGTCACCTGGTCCTTTTCACCTGTGCACAGGTAACCTCAGACTCGAGTCAA-3′, B reverse 5′-AGCTTTGACTCGAGTCTGAGGTTACCTGTGCACAGGTGAAAAGGACCAGGTGACCACGCTGGGTAC-3′; C forward 5′-CGAGCAAGCCCCAGGCAGCTACACTGGGCATGCTCA-3′, C reverse 5′-AGCTTGAGCATGCCCAGTGTAGCTGCCTGGGGCTTGCTCGGTAC-3′; and E forward 5′-CAACCGTGCAGCCTCTTCCTCGGCTTCTCCTGAAAGGGAAGGTGGAAGCCGTGGGCA-3′, E reverse 5′-AGCTTGCCCACGGCTTCCACCTTCCCTTTCAGGAGAAGCCGAGGAAGAGGCTGCACGGTTGGTAC-3′.
Chromatin immunoprecipitation assays (ChIP)
Chromatin immunoprecipitation was carried out using the ChIP-IT Express Kit as per the manufacturer's protocol (Active Motif, Carlsbad, CA, USA) and as described previously.34 Sequences for primers are PTEN A forward 5′-CGGGGTACCCGTCCCACTCACAGGGATCCTCT-3′ and reverse 5′-CCCAAGCTTCTGCTATTGTGTCGCCAGCGTCT-3′; PTEN B forward 5′-CCCTGCATTTCCTCTACA-3′ and reverse 5′-GAGGCGAGGATAACGAGCTA-3′; PTEN C forward 5′-GCACCCATCTCAGCTTTCAT-3′ and reverse 5′-CAAGCCGCAGGCTCTACT-3′; PTEN D forward 5′-GACTGCGCTCAGTTCTCTCC-3′ and reverse 5′-AGAGGCTGCACGGTTAGAAA-3′; and PTEN E forward 5′-CGGGGGTACCAGCGGCGGCGTTTCTCGCCTCCT-3′ and reverse 5′-CCCAAGCTTAGACCCCCTCCCTGGAGCTGCAG-3′; primers for the p21 promoter were as described previously.34 PCR conditions used are as follows: a total of 40 cycles were performed, each consisting of 30 s at 94°C, 30 s at 55°C and 30 s at 68°C.
Western blot analysis
Whole-cell extracts were prepared by lysing the cells in phosphatase inhibitors containing buffer (50 mM Tris-HCl pH 8, 120 mM NaCl, 5 mM NaPPi, 10 mM NaF, 30 mM paranitrophenylphosphate, 1 mM benzamidine, 0.1% NP-40, 1% Triton X-100 and 0.2 mM PMSF, 100 nM sodium orthovanadate) supplemented with protease inhibitors. Subcellular fractionations were carried out using the NE-PER kit as per the manufacturer's instructions (Pierce, Rockford, IL, USA). Immunoblot analysis was carried out as described previously.34
Antibodies
p63 antibodies used are as follows: pan-p63 4A4, p63alpha-isoform-specific H-129 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and ΔNp63-specific RR-14 and TAp63-specific antibodies (courtesy of Dr. Satrajit Sinha, SUNY Buffalo).40 The p53 antibody (Ab-6) was purchased from Calbiochem (Gibbstown, NJ, USA). PTEN (#9552), phospho-Akt (Ser 473, #9271), total Akt (#9272) and total PARP (#9542) antibodies were purchased from Cell Signaling (Danvers, MA, USA), whereas monoclonal anti-β-actin was purchased from Sigma Aldrich (St. Louis, MO, USA). Antibodies specific to total p73 (IMG-259A) and ΔNp73 (IMG-313A) were purchased from Imgenex (San Diego, CA, USA). The antibody for α-tubulin was graciously donated by Dr. Paula Bubulya (Wright State University). Appropriate horseradish peroxidase-conjugated secondary antibodies (Promega) were used for chemiluminescence detection with Supersignal Westpico Chemiluminescent Substrate kit (Pierce).
Cell growth assays
Cells harvested after a second round of siRNA transfection were re-seeded at 2 × 104 cells/well in a six-well plate for colony-formation assays. At 72 h, cells were stained with 1% crystal violet. A total of 20 random images were taken per condition, and colony number from each frame was measured using ImagePro 6.2 Software (Media Cybernetics, Bethesda, MD, USA). For cell growth assays, cells were re-seeded at 5000 cells/well in 96-well-plate MTS assays or at 10 000 cells/well in 12-well plates for trypan blue exclusion assays after 24 h from the second round of siRNA transfection. The MTS assay was carried out as per the manufacturer's instructions at the days indicated (Promega). Cell proliferation as measured by trypan blue exclusion was carried out in triplicate per condition per day post siRNA transfection.
Immunofluorescence
Human skin microarray (SK805, Biomax, Rockville, MD, USA) and paraffin-embedded embryonic mouse sections (a kind gift from Dr. Satrajit Sinha, SUNY Buffalo) were stained as previously described with minor modifications.34 Modifications included an antigen retrieval of 12 min in Tris/EDTA buffer (10 mM Tris, 1 mM EDTA, 0.05% Tween 20, pH 9.0) at 90°C in a pressure cooker and image was captured using a Leica CTR 6000 microscope (Leica Microsystems, Wetzlar, Germany) with ImagePro 6.2 Software (Media Cybernetics). AlexaFluor 488 and 568 secondary antibodies were used to visualize p63 and PTEN, respectively (Invitrogen). MFI for each tissue sample was calculated using ImagePro 6.2 software after normalization for background intensity. Multiple measurements (at least 5), all of the same size, were taken of the epidermal tissue for each tissue sample. All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.34 Human tissue samples were obtained and handled in full approval and accordance with Wright State University IRB protocols.
Statistical analyses
Statistical significance of differences was determined by Student's t-test for all experiments except MFIs, where the Pearson's product moment analysis was used to determine correlations between p63 and PTEN and significant difference was determined by one-way ANOVA with a Bonferroni's ad-hoc test. A probability value of <0.05 was considered statistically significant.
Accession codes
Abbreviations
- ΔNp63α:
-
deltaNp63alpha
- PTEN:
-
phosphatase and tensin homolog deleted on chromosome 10
- HEKs:
-
primary human epidermal keratinocytes
- RE:
-
response element
- CA-Akt:
-
constitutively active Akt
- BCC:
-
basal cell carcinoma
- SCC:
-
squamous cell carcinoma
- MFI:
-
mean fluorescence intensity
References
Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A . p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999; 398: 708–713.
Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 1998; 2: 305–316.
Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 2002; 416: 560–564.
Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA . The Delta Np63 alpha phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol 2003; 23: 2264–2276.
Barbieri CE, Perez CA, Johnson KN, Ely KA, Billheimer D, Pietenpol JA . IGFBP-3 is a direct target of transcriptional regulation by DeltaNp63alpha in squamous epithelium. Cancer Res 2005; 65: 2314–2320.
Wu G, Osada M, Guo Z, Fomenkov A, Begum S, Zhao M et al. DeltaNp63alpha up-regulates the Hsp70 gene in human cancer. Cancer Res 2005; 65: 758–766.
Choi HR, Batsakis JG, Zhan F, Sturgis E, Luna MA, El-Naggar AK . Differential expression of p53 gene family members p63 and p73 in head and neck squamous tumorigenesis. Hum Pathol 2002; 33: 158–164.
Sniezek JC, Matheny KE, Westfall MD, Pietenpol JA . Dominant negative p63 isoform expression in head and neck squamous cell carcinoma. Laryngoscope 2004; 114: 2063–2072.
Ogawa E, Okuyama R, Ikawa S, Nagoshi H, Egawa T, Kurihara A et al. p51/p63 inhibits ultraviolet B-induced apoptosis via Akt activation. Oncogene 2007; 27: 848–856.
Segrelles C, Moral M, Lara MF, Ruiz S, Santos M, Leis H et al. Molecular determinants of Akt-induced keratinocyte transformation. Oncogene 2006; 25: 1174–1185.
Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol 2000; 156: 1693–1700.
Whiteman DC, Zhou XP, Cummings MC, Pavey S, Hayward NK, Eng C . Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int J Cancer 2002; 99: 63–67.
Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK . Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol 2005; 25: 6211–6224.
Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y et al. Regulation of PTEN transcription by p53. Mol Cell 2001; 8: 317–325.
Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res 2002; 8: 494–501.
Mangiulli M, Valletti A, Caratozzolo MF, Tullo A, Sbisa E, Pesole G et al. Identification and functional characterization of two new transcriptional variants of the human p63 gene. Nucleic Acids Res 2009; 37: 6092–6104.
Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A et al. Subtle variations in Pten dose determine cancer susceptibility. Nat Genet 2010; 42: 454–458.
Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999; 398: 714–718.
Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW . p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 2006; 9: 45–56.
Strano S, Fontemaggi G, Costanzo A, Rizzo MG, Monti O, Baccarini A et al. Physical interaction with human tumor-derived p53 mutants inhibits p63 activities. J Biol Chem 2002; 277: 18817–18826.
Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C . A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol 2001; 21: 1874–1887.
Rolley N, Butcher S, Milner J . Specific DNA binding by different classes of human p53 mutants. Oncogene 1995; 11: 763–770.
Liu JL, Mao Z, LaFortune TA, Alonso MM, Gallick GE, Fueyo J et al. Cell cycle-dependent nuclear export of phosphatase and tensin homologue tumor suppressor is regulated by the phosphoinositide-3-kinase signaling cascade. Cancer Res 2007; 67: 11054–11063.
Parsa R, Yang A, McKeon F, Green H . Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol 1999; 113: 1099–1105.
Sakiz D, Turkmenoglu TT, Kabukcuoglu F . The expression of p63 and p53 in keratoacanthoma and intraepidermal and invasive neoplasms of the skin. Pathol Res Pract 2009; 205: 589–594.
Luukko K, Ylikorkala A, Tiainen M, Makela TP . Expression of LKB1 and PTEN tumor suppressor genes during mouse embryonic development. Mech Dev 1999; 83: 187–190.
Suzuki A, Itami S, Ohishi M, Hamada K, Inoue T, Komazawa N et al. Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation. Cancer Res 2003; 63: 674–681.
Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H . Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci USA 2006; 103: 1480–1485.
Vella V, Puppin C, Damante G, Vigneri R, Sanfilippo M, Vigneri P et al. DeltaNp73alpha inhibits PTEN expression in thyroid cancer cells. Int J Cancer 2009; 124: 2539–2548.
Yu J, Zhang SS, Saito K, Williams S, Arimura Y, Ma Y et al. PTEN regulation by Akt-EGR1-ARF-PTEN axis. EMBO J 2009; 28: 21–33.
Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M et al. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 201; 144: 187–199.
Bolshakov S, Walker CM, Strom SS, Selvan MS, Clayman GL, El-Naggar A et al. p53 mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin Cancer Res 2003; 9: 228–234.
Giglia-Mari G, Sarasin A . TP53 mutations in human skin cancers. Hum Mutat 2003; 21: 217–228.
Kommagani R, Leonard MK, Lewis S, Romano RA, Sinha S, Kadakia MP . Regulation of VDR by deltaNp63alpha is associated with inhibition of cell invasion. J Cell Sci 2009; 122 (Part 16): 2828–2835.
Mayo LD, Donner DB . A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA 2001; 98: 11598–11603.
Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills AA et al. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol 2006; 8: 551–561.
Chiang CT, Chu WK, Chow SE, Chen JK . Overexpression of delta Np63 in a human nasopharyngeal carcinoma cell line downregulates CKIs and enhances cell proliferation. J Cell Physiol 2009; 219: 117–122.
Guittet O, Tebbi A, Cottet MH, Vesin F, Lepoivre M . Upregulation of the p53R2 ribonucleotide reductase subunit by nitric oxide. Nitric Oxide 2008; 19: 84–94.
Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol 2001; 3: 1124–1128.
Romano RA, Birkaya B, Sinha S . A functional enhancer of keratin14 is a direct transcriptional target of deltaNp63. J Invest Dermatol 2007; 127: 1175–1186.
Acknowledgements
We thank the Kadakia laboratory members, particularly Kaitlyn Bunning, for technical assistance, and Dr. Satrajit Sinha and Dr. Elsa Flores for their gracious donations of materials. This work is funded by Boonshoft School of Medicine Seed Grant program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Leonard, M., Kommagani, R., Payal, V. et al. ΔNp63α regulates keratinocyte proliferation by controlling PTEN expression and localization. Cell Death Differ 18, 1924–1933 (2011). https://doi.org/10.1038/cdd.2011.73
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2011.73
Keywords
This article is cited by
-
∆Np63α inhibits Rac1 activation and cancer cell invasion through suppression of PREX1
Cell Death Discovery (2024)
-
A network map of cytoskeleton-associated protein 4 (CKAP4) mediated signaling pathway in cancer
Journal of Cell Communication and Signaling (2023)
-
ΔNp63α transcriptionally represses p53 target genes involved in the radiation-induced DNA damage response
Radiation Oncology (2022)
-
ERK3 is transcriptionally upregulated by ∆Np63α and mediates the role of ∆Np63α in suppressing cell migration in non-melanoma skin cancers
BMC Cancer (2021)
-
E47 upregulates ΔNp63α to promote growth of squamous cell carcinoma
Cell Death & Disease (2021)