Abstract
Postmenopausal osteoporosis (PMO) is a prevalent metabolic bone disease characterized by bone loss and structural destruction, which increases the risk of fracture in postmenopausal women. Owing to the high morbidity and serious complications of PMO, many efforts have been devoted to its prophylaxis and treatment. The intestinal microbiota is the complex community of microorganisms colonizing the gastrointestinal tract. Probiotics, which are dietary or medical supplements consisting of beneficial intestinal bacteria, work in concert with endogenous intestinal microorganisms to maintain host health. Recent studies have revealed that bone loss in PMO is closely related to host immunity, which is influenced by the intestinal microbiota. The curative effects of probiotics on metabolic bone diseases have also been demonstrated. The effects of the intestinal microbiota on bone metabolism suggest a promising target for PMO management. This review seeks to summarize the critical effects of the intestinal microbiota and probiotics on PMO, with a focus on the molecular mechanisms underlying the pathogenic relationship between bacteria and host, and to define the possible treatment options.
Similar content being viewed by others
Introduction
Postmenopausal osteoporosis (PMO) is an estrogen deficiency-induced metabolic bone disorder characterized by reduced bone strength, which increases the risk of fracture in postmenopausal women.1 The onset of PMO is occult, without any obvious symptoms until a fracture occurs. The most prevalent complication is a fragility fracture, which often occurs in the hip, femur, or spine under non-traumatic or mildly traumatic conditions, resulting in pain, malformation, dysfunction, and even death. Studies showed that the mortality rate associated with a hip fracture was 17% in the first year2 and ~12%–20% within the two following years.3 PMO is also a potential risk factor for oral bone loss and aggressive periodontitis in postmenopausal females. PMO animal models showed an equivalent bone loss in alveolar bone and femurs.4 Compared with healthy postmenopausal women, patients afflicted with PMO also exhibited an inclination to more bone loss and lower bone mineral density (BMD) in the jaw, especially in postmenopausal females with preexisting periodontitis who suffered from accelerated alveolar bone loss under routine treatment.5–7 In addition to bone loss and microstructural deterioration, PMO affects the osseous formation processes. Delayed osseous maturation and reduced bone regeneration during bone healing in ovariectomized (OVX) rats were reported.8,9 The high morbidity and serious complications of PMO have attracted major research efforts on its prophylaxis and treatment for decades. Current medications for the treatment of PMO include bisphosphonates, raloxifene, teriparatide and calcitonin, denosumab, estrogen and menopausal hormone therapy, and so on. These medications can prevent bone loss and increase bone mineral density, with a decreased risk of fractures in the vertebra, hip, or long bones.1,10 All of these pharmacological agents can reduce bone resorption by inhibiting osteoclasts, except teriparatide, which acts as an anabolic agent by activating or increasing osteoblast activity and prompting bone formation.1,11 Recent studies have demonstrated a close relationship between the intestinal microbiota and bone metabolism,12–15 providing evidence that the intestinal microbiome may serve as a potential therapeutic target for the treatment of PMO.
The intestinal microbiota and its regulators
The intestinal microbiota is the collection of microorganisms that colonize the gastrointestinal tract, which consists of approximately 10 trillion bacteria.16 Obligate anaerobes such as Bacteroidetes and Firmicutes are the predominant residents of the healthy gastrointestinal tract, outnumbering aerobes and facultative anaerobes.16,17 On the basis of their roles in maintaining human health, intestinal microorganisms can be categorized into beneficial, harmful and neutral bacteria. Both host and environmental factors can shape intestinal microbial composition and structure (Figure 1). Animal experiments18–21 and twin studies22,23 revealed that the host genetic background had a significant impact on the abundance of the intestinal microbiota and the predisposition to the colonization of pathogens (for example, Escherichia coli). Though still disputed, gender may be another host factor affecting intestinal microbiome species diversity.24,25 Environmental factors, including diet, lifestyle, hygiene, antibiotic treatment, and probiotics, also contribute to the alteration of the intestinal microbiota composition.26–31 Notably, the effects of diet and antibiotics on the intestinal microbiota also depend on the host genetic background.32,33
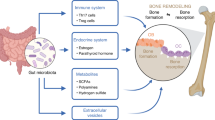
Regulators of the gut microbiota and mechanisms by which the gut microbiota regulates bone metabolism. Shaped by both host and environmental factors, the gut microbiota regulates bone metabolism through various pathways, including the immune system, endocrine system, and influences on calcium balance.
Probiotics are defined as dietary or medical supplements consisting of live bacteria that can benefit the host if provided in adequate quantities.34–36 Currently, ~20 types of beneficial bacteria are used in probiotic supplements. They are generally classified into five categories, including lactobacilli, bifidobacteria, streptococci, yeast, and others.37 Lactobacilli and bifidobacteria are the most commonly used probiotics. Probiotics can selectively ferment prebiotics, which contain soluble dietary fibers such as oligosaccharides and inulin, facilitating the production of beneficial products conducive to the growth of certain probiotics such as bifidobacteria.34,38,39 However, it is still disputed whether probiotics can alter the gut microbiota composition. Randomized controlled trials (RCTs) in healthy adults indicated that probiotic intervention or probiotics-fermented products resulted in changes in intestinal microbiota composition or diversity.40–43 Although probiotics promoted the significant increase of certain bacteria, Bacteroides was the dominant genus under probiotics administration, while other bacteria such as Clostridiales were inhibited.40,41 In addition, the effect of probiotics on Clostridiales genera may be associated with the initial status of the intestinal microbiome and butyrate concentrations.41 RCTs in elder adults showed that the age-associated intestinal microbiota imbalance was restored by probiotic-based functional foods, with increased resident probiotic-related bacteria and decreased emergence of opportunistic pathogens.44,45 Animal experimentation also showed that probiotic administration improved the intestinal microbiota composition in hyperlipidemic rats by recovering the abundance of Bacteroidetes and Verrucomicrobia and reducing Firmicutes.46 However, another RCT in healthy adults demonstrated that Lactobacillus rhamnosus GG (LGG) supplement induced no alteration in gut microbiota composition or diversity stability, except for a transient increased fecal excretion of probiotic-associated bacteria during the intervention.47 In addition, one RCT in healthy subjects and patients with irritable bowel syndrome (IBS) showed parallel, transient, and distinct increases in probiotics but limited changes in other specific bacteria in fecal samples of both healthy and IBS-afflicted subjects with Bifidobacterium infantis intervention.48
The intestinal microbiota regulates bone metabolism
Involvement of the intestinal microbiota in bone metabolism
The dynamic homeostasis of the gut microbiome is critical to health. Accumulating evidence has demonstrated that the gut microbiota is associated with physiological bone metabolism and a range of inflammatory or metabolic bone diseases.12–15,49,50 In animal experimentation, germ-free mice showed higher trabecular volume bone mineral density (vBMD) and improved histomorphologic indices in trabecula compared with conventionally raised (CONV-R) mice.12 However, both trabecular BMD and cortical cross-sectional area decreased when germ-free mice were recolonized by the gut microbiota, indicating that the gut microbiota is a major regulator of bone mass.12 Microbial recolonization in germ-free mice induced an incipient acute decrease in bone mass but predominantly led to bone formation with a longer duration, leading to a new equilibrium in bone mass.14 Furthermore, germ-free mice colonized with immature gut microbiota from donors of different ages or nutritional statuses showed varied femoral phenotypes, suggesting that the impact of the gut microbiota on bone morphologic properties is age/nutrition dependent.13 Compromised bone biomechanical properties in mice was also induced by an altered gut microbiota resulting from immunodeficiency or long-term antibiotic intervention during growth.15 In addition, through post-weaning exposure to low-dose penicillin (LDP) or by introducing LDP to their mother in pregnancy, adult offspring with a perturbed gut microbiota showed altered bone mineral content (BMC) and BMD.51 In addition to physiological condition, inflammatory, or metabolic bone diseases, such as metabolic osteoarthritis, osteoporosis, autoinflammatory osteomyelitis, are also associated with gut microbial alteration.49,50,52,53 The abundance of gut bacteria Lactobacillus spp. and Methanobrevibacter spp. was shown to have a significant relationship with the prediction of osteoarthritis assessed by the Modified Mankin Score in rats.50 Gut microbiota modified by diet regulated the production of IL-1β (Interleukin-1beta) and prevented the spontaneous development of osteomyelitis in Pstpip2cmo mice predisposed to autoinflammatory osteomyelitis.52,53
Mechanisms by which the gut microbiota regulates bone metabolism
Gut microbiota can regulate bone metabolism, but the exact mechanisms are still unclear. Multiple approaches through which gut microbiota may regulate bone metabolism have been proposed, including actions on the immune system, endocrine system, and calcium absorption (Figure 1).
(a) The gut microbiota regulates bone metabolism through the immune system
Recent studies have revealed a close interrelationship between the immune system and bone metabolism, leading to the development of “osteoimmunology,” which highlights the role of immune-related factors in modulating bone remodeling.54,55 In immune-mediated bone metabolism, the RANKL (receptor activator NF kappa B ligand)-RANK-OPG axis and immunoreceptor tyrosine-based activation motif (ITAM) pathway play key roles in physiological bone turnover and bone diseases.54,56 Recently, it has been widely recognized that the gut microbiota can interact with the host immune system and further influence host health.57–59 One study showed that altered immune status in germ-free mice (for example, decreased pro-inflammatory cytokines, fewer CD4+ T cells and reduced osteoclast/precursor cells in bone marrow) may account for the higher bone mass than in CONV-R mice.12 Intestinal segmented filamentous bacteria in mice were shown to promote the production of IL-17 and IFN-γ (Interferon-gamma), both of which played critical roles in the formation of osteoclasts and osteoblasts.60–62 These studies suggest that the gut microbiota regulates bone metabolism by altering host immune status.
(b) The gut microbiota regulates bone metabolism through the endocrine system
In addition to the immune system, hormones are regarded as another important regulator of bone metabolism. As an autocrine or paracrine growth factor, insulin-like growth factor-1 (IGF-1) can promote the differentiation and growth of bone cells, including osteoblasts, osteoclasts, and chondrocytes, and enhance normal interactions among them.63–65 Moreover, the IGF-1 signaling pathway is involved in the regulation of bone metabolism via both growth hormone and parathormone.64 Intermittent administration of parathormone promoted bone formation by increasing local IGF-1 production and activating the IGF-1 signaling pathway in bone.64 Growth hormone can directly or IGF-1-dependently target the growth plate to promote cartilage formation and longitudinal bone growth.66,67 Moreover, gonadal steroids, including estrogen and androgen, play key roles in the regulation of bone mass and turnover in bone metabolism.68–70 Furthermore, serum neurotransmitter 5-hydroxytryptamine, namely, circulating serotonin with a hormone-like effect, can stimulate or inhibit bone formation, and dual-directional effects may be gender/age dependent.71–74 The gut microbiota, which is currently considered a novel “endocrine organ” of the human body, can engage in an interplay with the endocrine system (for example, hypothalamic–pituitary–adrenal axis) and secrete hormones or hormone-like products to regulate host hormone levels, further influencing host health status.75,76 In animal experimentation, gut microbial colonization in germ-free mice significantly increased the serum IGF-1 level, resulting in bone growth and normalized bone mass.14 Isoflavones, the compounds classified as phytoestrogens and structurally similar to endogenous estrogen, were converted into more the estrogenic metabolite equol by specific gut microorganisms such as rod-shaped and gram-positive anaerobic bacteria in ~30%–50% of humans.77–81 Polycyclic aromatic hydrocarbons—contaminants widely present in nature—can be bio-transformed into products with estrogenic activity by the human colon microbiota.82 A recent study showed that the gut microbiota, especially spore-forming bacteria, can enhance the biosynthesis of serotonin by colonic enterochromaffin cells.83 Despite the lack of direct evidence, it has been suggested that gut microbiota-bone communication likely depends on the endocrine system or hormone-like substances.
(c) The gut microbiota regulates bone metabolism by influencing calcium absorption
Gut microbiota can affect the absorption of skeletal development-related nutrients such as calcium and vitamin D. Calcium, the dominant mineral component in bone, is essential for bone health. Calcium absorption can be facilitated by vitamin D. Either dietary calcium deprivation or vitamin D deficiency may induce osteoporosis.84 Sufficient calcium consumption can be a prophylactic measure against osteoporosis and relevant fracture.85 A clinical study in adolescent girls showed decreased bone resorption in the presence of high calcium consumption (47.4 mmol per day compared to the recommended 22.5 mmol per day).86 Some studies showed that calcium metabolism differences among ethnic groups—in terms of dietary calcium intake, renal calcium excretion, and relevant regulatory hormone or factor—were associated with bone parameters related to osteoporosis/fracture risk.87 In animal models, a low-calcium diet alone can lead to bone resorption, high bone turnover, and impaired bone trabecular microarchitecture in multiple bones, including the hard palate, mandible, vertebrae, femur, and proximal tibia.88–91
Calcium is absorbed by the active transcellular pathway (ion pumps) or passive paracellular diffusion (ion channels), depending on the level of 1,25-(OH)2D (1,25-dihydroxy vitamin D).92 The proteins involved in the transcellular pathway consist of transient receptor potential vanilloid type 6 (TRPV6/CaT1/ECaC2), which absorbs calcium from the gut lumen into cells; calbindin-D9k, which is responsible for intracellular calcium transportation; and plasma membrane calcium ATPase 1b (PMCA1b), which excretes calcium outside cells into the blood.93 Passive paracellular calcium diffusion occurs as calcium (Ca2+) flux across the intestinal epithelium and is based on tight junction (TJ) proteins between intestinal epithelial cells.94 Normal calcium intake rates in adults are ~30%–35%;95,96 these levels can be increased by probiotics, prebiotics, and synbiotics consisting of probiotics and their favorable prebiotics.97 Specific probiotic bacteria, such as Lactobacillus salivarius rather than Bifidobacterium infantis, stimulated calcium uptake by enterocytes in a Caco-2 cell culture model.98 Oligosaccharides (NDO), the dietary prebiotics containing fructooligosaccharides (FOS) and inulin, significantly facilitated intestinal calcium absorption and increased skeletal calcium content in growing and adult rats.99–102 Prebiotic inulin produced an enhancement in calcium absorption compared to other oligosaccharides,99,100 while the combination of both may act synergistically.101,102 In addition, a study in healthy adolescent girls demonstrated that daily administration of GOS can increase calcium absorption.103 Another clinical study reported the improvement of calcium absorption in young healthy women with long-term treatment with lactosucrose.104
As the fermentation substrates of gut microbiota, prebiotics affect bone metabolism by producing a variety of beneficial metabolites, such as short-chain fatty acids (SCFA). The potential mechanism by which SCFA regulate bone metabolism involves direct effects on proteins associated with calcium absorption. Experiments both in vitro and in vivo using animal models showed that an SCFA supplement could increase the transcriptional levels of TRPV6 and calbindin-D9k rather than PMCA1b in cultured Caco-2 human colonic epithelium and rat colorectal mucosa.105,106 The TRPV6 gene was shown to contain a segment characterized by a positive response to SCFA.105 In addition, the response of calbindin-D9k to SCFA varied with time and SCFA dose.106 The upregulation of calbindin-D9k by prebiotic diet specifically occurred in the colorectal segment regardless of dietary calcium uptake and serum 1,25-(OH)2D level, and it was related to the transcription factors vitamin D receptor (VDR) and cdx-2.107–109 The SCFA butyrate resulting from the prebiotic diet can upregulate VDR, activate the cdx-2 promoter, and facilitate cdx-2 mRNA expression.110 Although direct evidence for the SCFA-related effect on intestinal paracellular calcium absorption is still absent, a ruminant model in which more than 50% of calcium absorption pre-intestinally occurs in the rumen manifested a dose-dependent promotion by SCFA on the ruminal calcium ion flux rate from mucosa to serum in the paracellular pathway.111,112 As stated above, both probiotics and prebiotics can influence intestinal epithelial permeability by regulating TJ protein expression and distribution, which possibly underlies the mechanism of prebiotic effects on paracellular calcium transport. In addition to direct action on the cellular structure involved in the calcium absorption process, prebiotics can also alter the intestinal microenvironment, thereby indirectly modulating bone metabolism. SCFA generated from prebiotics could lower the intestinal lumen pH and consequently inhibit the formation of calcium complexes, such as calcium phosphates, leading to increased calcium absorption.113
Relationship between the intestinal microbiota and PMO
PMO animal models
Current data on the relationship between intestinal microbiota and PMO are primarily obtained from animal models. The most commonly used PMO animal models are rodents submitted to either surgery or medication. Ovariectomy is the most frequently used surgery to generate PMO rodent models. Bilateral ovariectomy is used to successfully set up morbid states of PMO in the proximal tibia, distal femur and lumbar vertebra according to the guidelines for the preclinical and clinical evaluation of PMO medication issued by the United States Food and Drug Administration (FDA).114 Gonadotropin-releasing hormone (GnRH) agonists are frequently used to induce PMO in rodents. The long-term or high dose administration of GnRH agonists to rats typically housed under germ-free conditions 49 inhibits the secretion of endogenous GnRH, gonadotrophin and estrogen.115,116 GnRH agonist-induced bone loss is reversible. Kurabayashi et al found that Sprague-Dawley (SD) rats submitted to long-term GnRH agonist treatment exhibited decreased bone mass, bone density, and bone turnover that could be partially recovered after treatment interruption.115 Estrogen deficiency induced by either ovariectomy or GnRH agonist in murine models evidently increases bone turnover and bone loss and reduces bone mineral density and bone volume in lumbar vertebrae and long bones, thus recapitulating conditions in patients with PMO.114,115,117
Animal age can affect the final experimental results, as preadolescent mice undergo rapid bone growth and high bone turnover due to the presence of growth hormones.118 In addition, mice are likely to undergo irreversible aging symptoms119 and potentially develop senile osteoporosis as early as 5–6 months old. Therefore, 8- to 20-week-old rats or mice are usually used to establish PMO animal models.49,115,118,120–128
PMO development depends on the intestinal microbiota and host genetic background
The intestinal microbiota is indispensable to PMO development. Compared to conventionally raised (Con-R) mice, germ-free (GF) mice showed no significant alteration in either pro-inflammatory cytokines in bone marrow or femoral trabecular parameters after PMO model establishment by the administration of GnRH agonists.49 However, similar to Con-R mice, GF mice colonized with a normal gut microbiota exhibited increased pro-inflammatory cytokines and impaired bone properties due to estrogen deficiency.49 Accordingly, intestinal microorganisms are involved in estrogen deficiency-associated trabecular bone resorption. These microorganisms may be correlated with certain trabecular bone parameters. In particular, trabecular number (Tb.N) and trabecular spacing (Tb.Sp) are influenced by the intestinal microbiota, whereas trabecular thickness (Tb.Th) is not.49
Bone resorption in PMO has also been shown to be closely related to genetic background (Figure 2). Previous studies have shown that estrogen deficiency-induced bone loss varies remarkably among different mouse strains.124,126,127 Genetic regulation can act on PMO bone loss through multiple mechanisms. Genetic background determines basal bone mass1,122 and the specific distribution of intestinal antigen-presenting cells (APCs) with different functions.129 Intestinal APCs, especially dendritic cells (DCs), present pathogenic antigens from the gut microbiota and activate CD4+ T cells to produce pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), which stimulates osteoclastogenesis and induces bone loss.130,131 In addition, host genetic background can shape the intestinal microbiota,20,22,23,33,132,133 which can influence the development and activity of host immune systems 59,134 and thus may indirectly regulate bone loss in PMO.
Probiotics prevent bone loss in PMO murine models
Bone loss in PMO murine models can be prevented by probiotics. Several studies have shown that bone resorption of femur and vertebra in OVX mice could be completely inhibited by the administration of probiotics such as Lactobacillus reuteri, LGG and the commercial mixture VSL#3.49,118 In addition, probiotics such as Bifidobacterium longum, Lactobacillus paracasei and a mixture of Lactobacillus paracasei and Lactobacillus plantarum alleviated femoral bone loss and increased bone mineral density in OVX rats or mice.120,121 Furthermore, soy skim milk fermented by Lactobacillus paracasei subsp. paracasei NTU 101 (NTU 101F) and Lactobacillus plantarum NTU 102 (NTU 102F) mitigated bone loss and improved the trabecular microarchitecture in OVX mice.125
The effects of probiotics on bone tissues depend on the systemic conditions of the host. McCabe LR et al123 showed that L. reuteri increased trabecular bone parameters of the femur and vertebra in healthy male mice (but not intact female mice), suggesting that estrogen level might affect the sensitivity of bone formation to L. reuteri in mice. L. reuteri may affect bone metabolism by activating the estrogen signaling pathway in male mice, whereas healthy adult female mice are impervious to L. reuteri due to sufficient estrogen. Notably, probiotics enhanced the trabecular bone parameters in intact female mice under inflammatory conditions after surgery.49,135 These results indicate that inflammatory pathways may be potential targets of probiotics to normalize bone homeostasis.
Host and microbiota interactions in the pathogenesis and treatment of PMO
Immune responses mediated by antigens from the intestinal microbiota play a central role in the pathogenesis of PMO. Under healthy conditions, interplays between the intestinal microbiota, the intestinal epithelial barrier, and the host immune system maintain homeostasis, inhibiting the number of intestinal pathogens and maintaining musculoskeletal balance. If homeostasis is disturbed, intestinal pathogens intrude into the host through the epithelial barrier and provoke an immune response, ultimately promoting osteoclastic bone resorption and continual bone loss in PMO. Accordingly, probiotics ameliorate bone resorption and destruction by suppressing immune responses and restoring equilibrium between the intestinal microbiota and the host.
Intestinal microbial diversity in PMO is regulated by estrogen and probiotics
A healthy state and sufficient estrogen levels maintain intestinal microbial diversity (Figure 3a). Under these conditions, beneficial bacteria are predominant and stunt the growth of pathogenic species, preserving the stability of the intestinal microbiota composition. In postmenopausal women, the absence of estrogen alters intestinal microbial composition and structure, leading to decreased microbial diversity (Figure 3b). Clinical surveys of males and postmenopausal females have shown significant correlations between biodiversity (or Clostridium abundance) in feces and urinary levels of estrogen (or estrogen metabolites).136,137 Estrogen deficiency destroys intestinal microbial diversity, which is reflected as a reduction in Firmicutes populations, including Clostridium species.136–138 Firmicutes bacteria, especially Clostridium species, possess immune-regulatory effects that boost the formation of regulatory T cells (Tregs) and enhance their function, sustaining immune homeostasis.139,140 Hence, estrogen deficiency undermines intestinal microbial diversity and reduces the abundance of intestinal bacteria that are conducive to immune homeostasis, consequently facilitating pathogen reproduction and initiating an immune response.
Intestinal microbial diversity in PMO is regulated by estrogen and probiotics. Healthy status can maintain gut microbial diversity and beneficial bacteria, which can activate Tregs to sustain immune homeostasis that is resistant to pathogens (a). Estrogen deficiency reduces gut microbial diversity and beneficial bacteria, while increased pathogens induce inflammation (b). Probiotics can prevent pathogens and increase gut microbial diversity by producing extracellular substances (c).
When used to treat PMO, probiotics improve intestinal microbial constitution and restore biodiversity. Probiotics halt pathogen growth and increase intestinal microbial diversity by synthesizing extracellular compounds (Figure 3c). A study by Preidis GA et al141 showed that L. reuteri increased microbial diversity and homogeneity in the feces of mice by producing reuterin. Reuterin, an antibiotic compound, promotes oxidative stress in cells by inducing the modification of thiols on proteins or small molecules, which in turn suppress the growth of pathogens such as Bacteroides while increasing the presence of Clostridium species.118,142 Additionally, the Lactococcus lactis strain G50 prevented H2S-producing bacteria from growing, while strain H61 had an inhibitory effect on Staphylococcus in a mouse model of senile osteoporosis.119,143 However, it has not yet been demonstrated whether L. lactis has an equivalent role in PMO.
Intestinal epithelial barrier function in PMO is regulated by estrogen and probiotics
The intestinal epithelium is the first barrier to physically resist intestinal pathogens. This barrier not only absorbs water and nutrients but also limits the penetration of intestinal antigens. The ability of the barrier to function properly depends on transcellular and paracellular pathways. The fundamental paracellular pathway structure is the TJ, the integrity and selective permeability of which are of vital importance to intestinal epithelial barrier function. TJs are protein complexes consisting of claudin, occludin, and zonula occludens (ZO) proteins, which together allow selective passage of ions and small molecules.144–150 TJ permeability can be represented by transepithelial electrical resistance (TER); higher TER usually indicates lower permeability.151,152 Both physiological and pathological stimuli can affect the production and distribution of TJ proteins, thereby modulating intestinal epithelial permeability. TJ proteins are mainly regulated by phosphorylation through protein kinase A (PKA), protein kinase C (PKC), protein kinase G (PKG), serine/threonine (Ser/Thr) kinases, Rho, mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase/Akt (PI3K/Akt), and myosin light chain kinase (MLCK).144,150
Sufficient levels of estrogen activate the GTP-binding protein Ras and a series of kinases present in cytoplasm (Raf, MEK1/2, and ERK1/2) through estrogen receptors on the intestinal epithelium; they also maintain relatively high levels of occludin protein expression (Figure 4a).144,153–155 As a result of this paracellular pathway, the intestinal epithelial barrier exhibits increased TER and can prevent pathogen invasion. Estrogen deficiency weakens the effect of the aforementioned estrogen-associated pathway, leading to increased intestinal epithelial permeability.156 Antigens from intestinal pathogens initiate inflammatory cascades across the epithelial barrier, leading to the production of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ). TNF-α and IFN-γ downregulate the TJ proteins occludin and ZO-1 via Raf-MEK1/2-ERK1/2 or MLKs-MKK3/6-p38 in the MAPK pathway and further compromise the intestinal epithelial barrier.157 In addition, the pro-inflammatory factor interleukin-17 (IL-17) can increase claudin-1 protein expression and reinforce the intestinal epithelial barrier through Ras-Raf-MEK1/2-ERK1/2 in the MAPK pathway.158 However, the positive action of IL-17 fails to completely compensate for the adverse effect of TNF-α and IFN-γ because TNF-α and IFN-γ may be central players in the immune responses elicited by intestinal bacteria. Hence, estrogen deficiency increases intestinal epithelial permeability (Figure 4b), facilitating the intrusion of intestinal pathogens and provoking immune reactions, and ultimately resulting in increased osteoclastic bone resorption and continual bone loss in PMO.
Intestinal epithelial barrier function in PMO is regulated by estrogen and probiotics. Sufficient estrogen can prompt the expression of tight junction (TJ) proteins through the Raf-MEK1/2-ERK1/2 pathway to enhance the gut epithelial barrier (a), while this active effect on TJ is weakened by estrogen deficiency (b). Under estrogen deficiency, pathogen-induced pro-inflammatory cytokines such as TNF-α and IFN-γ reduce the production of TJ proteins through both the Raf-MEK1/2-ERK1/2 and MLKs-MKK3/6-p38 pathways and compromise the gut epithelial barrier (b). The positive action of IL-17 on TJ proteins (thin green arrows in b) fails to completely compensate for the adverse effect of TNF-α and IFN-γ. Probiotics can enhance the gut epithelial barrier by regulating the production and distribution of TJ proteins and affecting the growth and movement of intestinal epithelial cells (c).
When used to treat PMO, probiotics fortify the intestinal epithelial barrier to protect the host against intestinal pathogen invasion (Figure 4c). Probiotics regulate the production and distribution of TJ proteins and reduce intestinal epithelial permeability by inducing changes in TJ-related gene expression. In vitro experiments have confirmed that L. plantarum can promote the production and rearrangement of claudin-1, occludin and ZO-1 proteins in the Caco-2 human colon adenocarcinoma cell line in a dose-dependent manner.159,160 Bifidobacteria infantis was found to increase ZO-1 and occludin protein expression by inhibiting pro-inflammatory cytokines or through the secretion of polypeptide bioactive factors to augment Erk levels while decreasing p38 levels.161 The probiotic mixture VSL#3 also promoted the expression and redistribution of occludin, ZO-1, and claudin-1 proteins in a mouse model of acute colitis.162 The potential mechanism for the probiotic regulation of TJ proteins probably involves SCFAs as fermentation products, especially butyrate, which could stimulate the reorganization of TJ proteins and promote TJ assembly by up-regulating AMP-activated protein kinase (AMPK) activity in the Caco-2 cell model, resulting in increased TER and an enhanced intestinal epithelial barrier.163 In addition, probiotics affected the growth and movement of intestinal epithelial cells by altering gene expression related to protein synthesis, metabolism, cell adhesion and apoptosis.162,164 L. reuteri substantially promoted intestinal epithelial cell migration and proliferation and increased intestinal crypt depth, ultimately improving the absorptive function of the intestinal epithelial barrier.141 Both LGG and L. plantarum can stimulate the intestinal epithelium to produce physiological levels of reactive oxygen species (ROS), which act as a second messenger to activate the Erk/MAPK pathway and consequently lead to intestinal epithelial proliferation.165,166 Probiotics also offer resistance against the toxic effects produced by intestinal pathogens on the intestinal epithelium. Bifidobacteria reduce the production of autophagy-related proteins and further prevent intestinal epithelial autophagy triggered by endotoxins from gram-negative bacteria.167
Host immune responses in PMO are regulated by estrogen and the intestinal microbiota
The immune system is the final barrier to intestinal pathogen invasion and is also a critical target for PMO treatment. APCs in the intestinal lamina propria can be divided into dendritic cells (DCs) and macrophages.129 Although all macrophages and DCs can induce Foxp3+ Treg cell differentiation, macrophages with a higher T cell/APC ratio are more efficient than DCs.129 By contrast, DCs only partially induce Th17 cell differentiation.129 Treg cells are a subset of immunocytes with inhibitory effects on the differentiation and function of Th1, Th2, and Th17 cells.130 In addition, Treg cells can inhibit osteoclast formation by cell-to-cell contact via the cytotoxic T lymphocyte antigen (CTLA-4) or by secreting anti-inflammatory cytokines such as IL-4, IL-10, and transforming growth factor-β (TGF-β).168–171 Th17 cells, a subgroup of T cells, stimulate osteoclast formation and bone resorption by producing high levels of IL-17, RANKL, and TNF-α.172
Both adequate estrogen levels and intestinal microbial diversity are needed to maintain immune homeostasis (Figure 5a). Clostridium improves the aggregation, quantity and function of Treg cells to create an environment abundant in TGF-β, which consequently prevents osteoclastogenesis.139 Estrogen protects bone by down-regulating immune responses and modulating osteoblast/osteoclast equilibrium.173 Estrogen not only activates the apoptosis-promoting Fas/FasL pathway through direct interaction with osteoclasts 174–177 but also indirectly increases TGF-β production by Treg cells and decreases the production of TNF-a and RANKL by Th17 cells, ultimately promoting osteoclast apoptosis.131,168,169,171,178,179 Furthermore, estrogen exerts anti-apoptotic effects on osteoblasts and osteocytes through the ERK pathway.177,180
Host immune responses in PMO are regulated by estrogen and intestinal microbiota. Both beneficial gut bacteria and sufficient estrogen activate Tregs, which produce TGF-β to prevent osteoclastogenesis and induce osteoclast apoptosis; estrogen prompts osteoblast formation to improve bone mass and structure (a). Estrogen deficiency reduces osteoblast formation; the invasion of pathogens activates CD4+T cells including TH17, which mainly produce TNF-α to promote osteoclastogenesis, leading to bone loss and microstructural destruction (b). Probiotics can regulate immune responses by secreting small molecules such as SCFAs and histamine (c).
Estrogen deficiency and reduced intestinal biodiversity have negative effects on bone (Figure 5b). Pathogenic antigens cross the intestinal epithelium and trigger inflammatory immune responses that are mainly mediated by T cells. Estrogen deficiency boosts the antigen presentation of DCs and macrophages through multiple pathways. Upon estrogen depletion, ROS excessively accumulate in bone marrow cells.181,182 ROS enhance the antigen-presenting function of DCs, which further activates CD4+T cells to produce IFN-γ. The enhanced production of IFN-γ in turn improves the antigen-presenting ability of bone marrow macrophages (BMM) by up-regulating MHC II molecules.183–187 In addition, estrogen deficiency upregulates co-stimulator CD80 to activate bone marrow DCs.184 Increased antigen presentation motivates CD4+ cells, including IL-17-producing Th17 cells, to mediate osteoclast formation and bone resorption.130,188 In addition to antigen-dependent activation, increased levels of IFN-γ and IL-7, in combination with low levels of TGF-β, indirectly activate T cells in bone marrow.130,188,189 Activated T cells generate a considerable quantity of TNF-α, which acts as a key pathogenic factor in PMO development.131,190–193 TNF-α stimulates the production of RANKL and macrophage colony stimulatory factor (M-CSF); it also suppresses the production of osteoprotegerin (OPG) by inducing the expression of CD40L and the bone mass regulatory factor DLK1/FA-1.130,194,195 In addition, TNF-α acts either directly on osteoclast precursors to promote their maturation196 or indirectly on TNF-α receptor p55 to augment M-CSF- and RANKL-induced osteoclastogenesis.131 Furthermore, estrogen deficiency increases levels of Act1 adaptor protein on the surfaces of osteoblasts and subsequently activates the IL-17 signal pathway to promote bone resorption.197,198 These findings provide evidence that CD4+ T cells (including Th17 cells) and the pro-inflammatory cytokine TNF-α are primary factors responsible for bone loss mediated by intestinal bacteria in PMO.
When used for PMO treatment, probiotics also suppress bone resorption by regulating immune responses to intestinal microorganisms. Probiotics secrete small molecules to regulate the host immune response (Figure 5c). Probiotics also produce SCFAs by utilizing prebiotics.30,34,199,200 SCFA receptors contain GPR41 and GPR43, the latter of which is mainly found in immunocytes such as neutrophils and monocytes.201 SCFAs, especially butyric acid, interact with GPR43 to reduce levels of monocyte chemotactic protein 1 (MCP-1) and LPS-induced cytokines such as TNF-α and IFN-γ. They also up-regulate the expression of TGF-β1, IL-4 and IL-10, ultimately activating Treg cells.120,201–205 In addition, L. reuteri transforms dietary L-histidine to histamine, which inhibits the MEK1/2-ERK1/2 pathway via H2 receptors and further inhibits TNF-α production by monocytes.206 Lactobacillus also impedes DC activation during inflammation and promotes Treg differentiation by inducing the expression of molecular ligands with inhibitory effects on pertinent DNA motifs.207
The intestinal microbiota and estrogen orchestrate calcium absorption
As described above, both calcium content and estrogen level are critical to bone metabolism. In postmenopausal-osteoporotic rats, combined deficiencies of dietary calcium and estrogen had a more adverse effect on bone mass and microstructure than either single deficiency, with more bone loss and more severely impaired bone properties.88,90,208 In addition, calcium balance can be regulated by estrogen. Under normal conditions, estrogen treatment can increase intestinal calcium absorption in rats.209 Accumulating evidence suggests that estrogen deficiency could induce impaired calcium absorption, which was improved by estrogen supplementation.210–212 The potential mechanisms of estrogen-associated regulation on calcium absorption are still disputed. Estrogen may indirectly promote vitamin D receptor (VDR) protein expression and enhance intestinal mucosal responsiveness to 1,25-(OH)2D, resulting in increased intestinal calcium absorption.213,214 However, estrogen deficiency-related calcium malabsorption may not depend on the serum 1,25-(OH)2D pathway. Estrogen reversed the reduced calcium absorption by directly interacting with estrogen receptor alpha (ER-α) on the intestine, up-regulating the calcium transport protein 1 (CaT 1) of the calcium influx channel without significantly altering serum 1,25-(OH)2D level.212,215,216 In addition, estrogen deficiency increased the urinary fractional excretion of calcium (FECa) in OVX rats.120
The imbalance in calcium metabolism induced by estrogen deficiency was also redressed by the application of probiotics and prebiotics for the treatment of PMO.97 Probiotic supplements completely inhibited the increase in FECa due to estrogen deficiency in OVX rats.120 Oligosaccharides (NDO), dietary prebiotics such as fructooligosaccharides (FOS), galactooligosaccharides (GOS), and inulin, can significantly promote intestinal calcium absorption and skeletal calcium retention in OVX rats, resulting in suppressed bone loss.217,218
The gut microbiota produces estrogen-like metabolites with regulatory effects on bone metabolism
Estrogen plays a major role in promoting osteogenesis. The role of estrogen is not limited to the direct suppression of osteoclast activity and lifespan, facilitation of osteoblast lifespan and differentiation, or reduction of mature osteoblasts apoptosis to promote osteogenesis. It also inhibits the formation of both osteoblasts and osteoclasts from bone marrow precursors to prevent bone remodeling and regulate bone turnover.69,70 In the absence of estrogen due to ovariectomy or post-menopause, estrogen-deficient women exhibit accelerated bone loss and increased bone turnover as well as impaired bone microarchitectural and mechanical properties.49,177,219 Hormone replacement therapy (HRT), including supplementation with estrogen and progesterone, has been applied to postmenopausal women suffering from PMO and achieved favorable effects.220 Instead of estrogen supplementation, the gut microbiota may act as another “endocrine organ” and potentiate novel access to replenish estrogen by utilizing exogenous nutrients and producing more estrogenic substances.
Phytoestrogens, which are predominantly present in natural foods such as soy, are exogenous nutrients with structures and bioactivity similar to human intrinsic estrogens. Various metabolites produced from phytoestrogens by the gut microbiota, including equol, urolithins, and enterolignans, are characterized by higher bioavailability and respectively more estrogenic, antiestrogenic and antioxidant bioactivities than their precursors in phytoestrogens, such as isoflavones, ellagitannins, and lignans.221 Daidzein, the principle isoflavone in soy, has two metabolic patterns including equol and O-desmethylangolensin (O-DMA) production.222 Equol shows much more estrogenic bioactivity or effects than O-DMA for bone metabolism in PMO.223 Equol, which is mostly present as a glucuronide conjugate and binds to the estrogen receptor (ER), can suppress bone resorption, promote bone formation, and improve bone biomechanical and microstructural properties in subjects with PMO but has no impacts on bone in healthy early postmenopausal women.224–229 The potential mechanism that involves equol may prevent osteoclast formation, stimulate the proliferation and differentiation of osteoblasts, and increase osteocalcin level by ER.223,230 Additionally, equol can inhibit the expression of relevant inflammatory cytokines in bone marrow in a dose-dependent fashion due to estrogen deficiency or LPS from intestinal pathogens.231–233 Although produced by gut microbiota, equol may modify gut microbiota diversity and composition in turn.231 Isoflavone metabolism can promote the growth of Clostridium clusters XIVa and IV and suppress the genera Bacteroides and Parabacteroides.234 Nevertheless, equol production from dietary phytoestrogens has significant interpersonal variations, predominantly depending on gut microbial composition and potential correlations among the three groups of phytoestrogen metabolism as well as dietary components.77,221,235,236 At present, the key equol-producing gut bacteria have not yet been identified. Most studies target potential equol-producing bacteria by cultivation or sequence analysis of fecal samples. Two strains of Eubacterium sp. were isolated and considered the most likely equol-producing bacteria from pig feces.237 Another intestinal bacteria, Slackia TM-30, a rod-shaped and gram-positive anaerobe isolated from healthy human feces, also proved to be highly related to equol production.78 The sequence information for fecal samples in postmenopausal women with dietary isoflavone uptake indicated obviously higher proportions of Eubacterium and Bifidobacterium in equol-producing subjects than in equol non-producers.238 Other studies identified other bacteria that significantly increased in fecal samples of equol producers, including Collinsella, Asaccharobacter, Dorea, and Finegoldia.234,239 In terms of function, sulfate-reducing bacteria were suggested to be involved in equol production.235 In addition to specific gut bacteria, equol-producing capacity may inversely correlate with O-DMA production.235 In addition, daidzein bioavailability and the equol/O-DMA production ratio could be elevated by the combined administration of isoflavones and prebiotic oligosaccharides or probiotic bacteria such as Lactobacillus casei.240–242 However, another study showed that the combination of soy isoflavones and fructooligosaccharides had no synergistic effects on bone mineral density or bone mineral content but effectively improved bone microstructural properties, including trabecular number, thickness, and separation.243 Overall, the beneficial effects of phytoestrogen supplementation on PMO mainly depend on individual metabolisms involving both the appropriate gut microbiome and dietary composition.244
Conclusion
Bone resorption in PMO is the consequence of interactions among the estrogen level, the intestinal microbiota, and the host immune system. When estrogen levels are deficient, bacteria and intestinal antigens cross the compromised intestinal epithelium barrier and initiate the immune responses associated with bone loss in PMO. Probiotics prevent bone resorption by restoring intestinal microbial diversity, enhancing the intestinal epithelial barrier, and normalizing aberrant host immune responses, as well as facilitating intestinal calcium absorption and the potential production of estrogen-like metabolites, as summarized in Table 1. Hence, the intestinal microbiota serves as a key factor in the pathogenesis of PMO and will also serve as a new target in the treatment of PMO.
The application of probiotics may be a promising adjuvant to current therapies. However, current studies on probiotics for PMO treatment are limited to animal studies. The translation from animal studies to clinical application faces many challenges, such as effective dosage and safety in humans. The safety and feasibility of probiotics application in humans have been demonstrated by clinical studies in specific groups, such as in healthy infants,245 preterm infants, children with intractable diarrhea,246 and children and adolescents undergoing HCT.247 However, in patients with predicted severe acute pancreatitis, significant increases in bowel ischemia and mortality were related to probiotic prophylaxis, as reported in the study by Besselink et al248 Hence, more studies are needed to validate the safety of probiotics and confirm the optimal dosage and the proper time and method of delivery for probiotics in the context of PMO treatment.
References
Watts NB, Bilezikian JP, Camacho PM et al. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010; 16 (Suppl 3): 1–37.
Forsen L, Sogaard AJ, Meyer HE et al. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int 1999; 10: 73–78.
Orwig DL, Chan J, Magaziner J . Hip fracture and its consequences: differences between men and women. Orthop Clin North Am 2006; 37: 611–622.
Macari S, Duffles LF, Queiroz-Junior CM et al. Oestrogen regulates bone resorption and cytokine production in the maxillae of female mice. Arch Oral Biol 2015; 60: 333–341.
Esfahanian V, Shamami MS, Shamami MS . Relationship between osteoporosis and periodontal disease: review of the literature. J Dent (Tehran) 2012; 9: 256–264.
LaMonte MJ, Hovey KM, Genco RJ et al. Five-year changes in periodontal disease measures among postmenopausal females: the Buffalo OsteoPerio study. J Periodontol 2013; 84: 572–584.
Passos JS, Vianna MI, Gomes-Filho IS et al. Osteoporosis/osteopenia as an independent factor associated with periodontitis in postmenopausal women: a case-control study. Osteoporos Int 2013; 24: 1275–1283.
Calciolari E, Mardas N, Dereka X et al. The effect of experimental osteoporosis on bone regeneration: part 2, proteomics results. Clin Oral Implants Res 2017; 28: e135–e145.
Durao SF, Gomes PS, Colaco BJ et al. The biomaterial-mediated healing of critical size bone defects in the ovariectomized rat. Osteoporos Int 2014; 25: 1535–1545.
Black DM, Rosen CJ . Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med 2016; 374: 254–262.
Deal C . Potential new drug targets for osteoporosis. Nat Clin Pract Rheumatol 2009; 5: 20–27.
Sjogren K, Engdahl C, Henning P et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res 2012; 27: 1357–1367.
Blanton LV, Charbonneau MR, Salih T et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016; 351. pii: aad3311.
Yan J, Herzog JW, Tsang K et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA 2016; 113: E7554–e7563.
Guss JD, Horsfield MW, Fontenele FF et al. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res 2017; 32: 1343–1353.
Qin J, Li R, Raes J et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464: 59–65.
Tojo R, Suarez A, Clemente MG et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol 2014; 20: 15163–15176.
Org E, Parks BW, Joo JW et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res 2015; 25: 1558–1569.
Kovacs A, Ben-Jacob N, Tayem H et al. Genotype is a stronger determinant than sex of the mouse gut microbiota. Microb Ecol 2011; 61: 423–428.
Benson AK, Kelly SA, Legge R et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci USA 2010; 107: 18933–18938.
Esworthy RS, Smith DD, Chu FF . A strong impact of genetic background on gut microflora in mice. Int J Inflam 2010; 2010: 986046.
Goodrich JK, Waters JL, Poole AC et al. Human genetics shape the gut microbiome. Cell 2014; 159: 789–799.
Turnbaugh PJ, Hamady M, Yatsunenko T et al. A core gut microbiome in obese and lean twins. Nature 2009; 457: 480–484.
Zhao L, Wang G, Siegel P et al. Quantitative genetic background of the host influences gut microbiomes in chickens. Sci Rep 2013; 3: 1163.
Dabrowska K, Witkiewicz W . Correlations of host genetics and gut microbiome composition. Front Microbiol 2016; 7: 1357.
Kashtanova DA, Popenko AS, Tkacheva ON et al. Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition 2016; 32: 620–627.
Schmidt B, Mulder IE, Musk CC et al. Establishment of normal gut microbiota is compromised under excessive hygiene conditions. PLoS One 2011; 6: e28284.
Panda S, El khader I, Casellas F et al. Short-term effect of antibiotics on human gut microbiota. PLoS One 2014; 9: e95476.
Cho I, Yamanishi S, Cox L et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488: 621–626.
Gibson GR, Roberfroid MB . Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995; 125: 1401–1412.
Shin JH, Sim M, Lee JY et al. Lifestyle and geographic insights into the distinct gut microbiota in elderly women from two different geographic locations. J Physiol Anthropol 2016; 35: 31.
Fujisaka S, Ussar S, Clish C et al. Antibiotic effects on gut microbiota and metabolism are host dependent. J Clin Invest 2016; 126: 4430–4443.
Leamy LJ, Kelly SA, Nietfeldt J et al. Host genetics and diet, but not immunoglobulin A expression, converge to shape compositional features of the gut microbiome in an advanced intercross population of mice. Genome Biol 2014; 15: 552.
Sekhon BS, Jairath S . Prebiotics, probiotics and synbiotics: an overview. J Pharm Educ Res 2010; 1: 13–36.
Roberfroid MB . Prebiotics and probiotics: are they functional foods? Am J Clin Nutr 2000; 71: 1682S–1687S.
Salminen S, Bouley C, Boutron-Ruault MC et al. Functional food science and gastrointestinal physiology and function. Br J Nutr 1998; 80 (Suppl 1): S147–S171.
Gupta V, Garg R . Probiotics. Indian J Med Microbiol 2009; 27: 202–209.
Roberfroid MB . Prebiotics and synbiotics: concepts and nutritional properties. Br J Nutr 1998; 80: S197–S202.
Roberfroid M, Gibson GR, Hoyles L et al. Prebiotic effects: metabolic and health benefits. Br J Nutr 2010; 104 (Suppl 2): S1–63.
Plaza-Diaz J, Fernandez-Caballero JA, Chueca N et al. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients 2015; 7: 3999–4015.
Ferrario C, Taverniti V, Milani C et al. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr 2014; 144: 1787–1796.
Zhang J, Wang L, Guo Z et al. 454 pyrosequencing reveals changes in the faecal microbiota of adults consuming Lactobacillus casei Zhang. FEMS Microbiol Ecol 2014; 88: 612–622.
Unno T, Choi JH, Hur HG et al. Changes in human gut microbiota influenced by probiotic fermented milk ingestion. J Dairy Sci 2015; 98: 3568–3576.
Rampelli S, Candela M, Severgnini M et al. A probiotics-containing biscuit modulates the intestinal microbiota in the elderly. J Nutr Health Aging 2013; 17: 166–172.
Ahmed M, Prasad J, Gill H et al. Impact of consumption of different levels of Bifidobacterium lactis HN019 on the intestinal microflora of elderly human subjects. J Nutr Health Aging 2007; 11: 26–31.
Chen D, Yang Z, Chen X et al. The effect of Lactobacillus rhamnosus hsryfm 1301 on the intestinal microbiota of a hyperlipidemic rat model. BMC Complement Altern Med 2014; 14: 386.
Lahti L, Salonen A, Kekkonen RA et al. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. Peer J 2013; 1: e32.
Charbonneau D, Gibb RD, Quigley EM . Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes 2013; 4: 201–211.
Li JY, Chassaing B, Tyagi AM et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 2016; 126: 2049–2063.
Collins KH, Paul HA, Reimer RA et al. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthritis Cartilage 2015; 23: 1989–1998.
Cox LM, Yamanishi S, Sohn J et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158: 705–721.
Phillips FC, Gurung P, Kanneganti TD . Microbiota and caspase-1/caspase-8 regulate IL-1beta-mediated bone disease. Gut Microbes 2016; 7: 334–341.
Lukens JR, Gurung P, Vogel P et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 2014; 516: 246–249.
Crotti TN, Dharmapatni AA, Alias E et al. Osteoimmunology: major and costimulatory pathway expression associated with chronic inflammatory induced bone loss. J Immunol Res 2015;2015: 281287.
Zupan J, Jeras M, Marc J . Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem Med (Zagreb) 2013; 23: 43–63.
Kim N, Takami M, Rho J et al. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med 2002; 195: 201–209.
Geuking MB, Koller Y, Rupp S et al. The interplay between the gut microbiota and the immune system. Gut Microbes 2014; 5: 411–418.
Peterson CT, Sharma V, Elmen L et al. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol 2015; 179: 363–377.
Palm NW, de Zoete MR, Flavell RA . Immune-microbiota interactions in health and disease. Clin Immunol 2015; 159: 122–127.
Ivanov II, Atarashi K, Manel N et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139: 485–498.
Adamopoulos IE, Chao CC, Geissler R et al. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther 2010; 12: R29.
Duque G, Huang DC, Dion N et al. Interferon-gamma plays a role in bone formation in vivo and rescues osteoporosis in ovariectomized mice. J Bone Miner Res 2011; 26: 1472–1483.
Wang Y, Nishida S, Elalieh HZ et al. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res 2006; 21: 1350–1358.
Wang Y, Bikle DD, Chang W . Autocrine and paracrine actions of IGF-I signaling in skeletal development. Bone Res 2013; 1: 249–259.
Wang Y, Nishida S, Sakata T et al. Insulin-like growth factor-I is essential for embryonic bone development. Endocrinology 2006; 147: 4753–4761.
Wu S, Yang W, De Luca F . Insulin-like growth factor-independent effects of growth hormone on growth plate chondrogenesis and longitudinal bone growth. Endocrinology 2015; 156: 2541–2551.
Giustina A, Mazziotti G, Canalis E . Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev 2008; 29: 535–559.
Leder B . Gonadal steroids and bone metabolism in men. Curr Opin Endocrinol Diabetes Obes 2007; 14: 241–246.
Imai Y, Youn MY, Kondoh S et al. Estrogens maintain bone mass by regulating expression of genes controlling function and life span in mature osteoclasts. Ann NY Acad Sci 2009; 1173 (Suppl 1): E31–E39.
Syed F, Khosla S . Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 2005; 328: 688–696.
Bliziotes M . Update in serotonin and bone. J Clin Endocrinol Metab 2010; 95: 4124–4132.
Wang Q, Chen D, Nicholson P et al. The associations of serum serotonin with bone traits are age- and gender-specific. PLoS One 2014; 9: e109028.
Gustafsson BI, Thommesen L, Stunes AK et al. Serotonin and fluoxetine modulate bone cell function in vitro . J Cell Biochem 2006; 98: 139–151.
Battaglino R, Fu J, Spate U et al. Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res 2004; 19: 1420–1431.
Neuman H, Debelius JW, Knight R et al. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev 2015; 39: 509–521.
Sudo N . Microbiome, HPA axis and production of endocrine hormones in the gut. Adv Exp Med Biol 2014; 817: 177–194.
Landete JM, Arques J, Medina M et al. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit Rev Food Sci Nutr 2016; 56: 1826–1843.
Tamura M, Hori S, Nakagawa H et al. Effects of an equol-producing bacterium isolated from human faeces on isoflavone and lignan metabolism in mice. J Sci Food Agric 2016; 96: 3126–3132.
Bowey E, Adlercreutz H, Rowland I . Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem Toxicol 2003; 41: 631–636.
Cassidy A, Brown JE, Hawdon A et al. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr 2006; 136: 45–51.
Song KB, Atkinson C, Frankenfeld CL et al. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr 2006; 136: 1347–1351.
Van de Wiele T, Vanhaecke L, Boeckaert C et al. Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environ Health Perspect 2005; 113: 6–10.
Yano JM, Yu K, Donaldson GP et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015; 161: 264–276.
Morris HA, O'Loughlin PD, Anderson PH . Experimental evidence for the effects of calcium and vitamin D on bone: a review. Nutrients 2010; 2: 1026–1035.
Zhu K, Prince RL . Calcium and bone. Clin Biochem 2012; 45: 936–942.
Wastney ME, Martin BR, Peacock M et al. Changes in calcium kinetics in adolescent girls induced by high calcium intake. J Clin Endocrinol Metab 2000; 85: 4470–4475.
Redmond J, Jarjou LM, Zhou B et al. Ethnic differences in calcium, phosphate and bone metabolism. Proc Nutr Soc 2014; 73: 340–351.
Hodgkinson A, Aaron JE, Horsman A et al. Effect of oophorectomy and calcium deprivation on bone mass in the rat. Clin Sci Mol Med 1978; 54: 439–446.
Hara T, Sato T, Oka M et al. Effects of ovariectomy and/or dietary calcium deficiency on bone dynamics in the rat hard palate, mandible and proximal tibia. Arch Oral Biol 2001; 46: 443–451.
Shen V, Birchman R, Xu R et al. Short-term changes in histomorphometric and biochemical turnover markers and bone mineral density in estrogen-and/or dietary calcium-deficient rats. Bone 1995; 16: 149–156.
Goto S, Fujita Y, Hotta M et al. Influence of differences in the hardness and calcium content of diets on the growth of craniofacial bone in rats. Angle Orthod 2015; 85: 969–979.
Fleet JC, Schoch RD . Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci 2010; 47: 181–195.
Hoenderop JG, Nilius B, Bindels RJ . Calcium absorption across epithelia. Physiol Rev 2005; 85: 373–422.
Alexander RT, Rievaj J, Dimke H . Paracellular calcium transport across renal and intestinal epithelia. Biochem Cell Biol 2014; 92: 467–480.
Kuwabara A, Tanaka K . The role of gastro-intestinal tract in the calcium absorption. Clin Calcium 2015; 25: 1607–1612.
Sheikh MS, Schiller LR, Fordtran JS . In vivo intestinal absorption of calcium in humans. Miner Electrolyte Metab 1990; 16: 130–146.
Scholz-Ahrens KE, Ade P, Marten B et al. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr 2007; 137: 838s–846s.
Gilman J, Cashman KD . The effect of probiotic bacteria on transepithelial calcium transport and calcium uptake in human intestinal-like Caco-2 cells. Curr Issues Intest Microbiol 2006; 7: 1–5.
Kruger MC, Brown KE, Collett G et al. The effect of fructooligosaccharides with various degrees of polymerization on calcium bioavailability in the growing rat. Exp Biol Med (Maywood) 2003; 228: 683–688.
Nzeusseu A, Dienst D, Haufroid V et al. Inulin and fructo-oligosaccharides differ in their ability to enhance the density of cancellous and cortical bone in the axial and peripheral skeleton of growing rats. Bone 2006; 38: 394–399.
Coudray C, Tressol JC, Gueux E et al. Effects of inulin-type fructans of different chain length and type of branching on intestinal absorption and balance of calcium and magnesium in rats. Eur J Nutr 2003; 42: 91–98.
Demigne C, Jacobs H, Moundras C et al. Comparison of native or reformulated chicory fructans, or non-purified chicory, on rat cecal fermentation and mineral metabolism. Eur J Nutr 2008; 47: 366–374.
Whisner CM, Martin BR, Schoterman MH et al. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Br J Nutr 2013; 110: 1292–1303.
Teramoto F, Rokutan K, Sugano Y et al. Long-term administration of 4G-beta-D-galactosylsucrose (lactosucrose) enhances intestinal calcium absorption in young women: a randomized, placebo-controlled 96-wk study. J Nutr Sci Vitaminol (Tokyo) 2006; 52: 337–346.
Fukushima A, Aizaki Y, Sakuma K . Short-chain fatty acids induce intestinal transient receptor potential vanilloid type 6 expression in rats and Caco-2 cells. J Nutr 2009; 139: 20–25.
Fukushima A, Aizaki Y, Sakuma K . Short-chain fatty acids increase the level of calbindin-D9k messenger RNA in Caco-2 cells. J Nutr Sci Vitaminol (Tokyo) 2012; 58: 287–291.
Fukushima A, Ohta A, Sakai K et al. Expression of calbindin-D9k, VDR and Cdx-2 messenger RNA in the process by which fructooligosaccharides increase calcium absorption in rats. J Nutr Sci Vitaminol (Tokyo) 2005; 51: 426–432.
Ohta A, Motohashi Y, Ohtsuki M et al. Dietary fructooligosaccharides change the concentration of calbindin-D9k differently in the mucosa of the small and large intestine of rats. J Nutr 1998; 128: 934–939.
Takasaki M, Inaba H, Ohta A et al. Dietary short-chain fructooligosaccharides increase calbindin-D9k levels only in the large intestine in rats independent of dietary calcium deficiency or serum 1,25 dihydroxy vitamin D levels. Int J Vitam Nutr Res 2000; 70: 206–213.
Domon-Dell C, Wang Q, Kim S et al. Stimulation of the intestinal Cdx2 homeobox gene by butyrate in colon cancer cells. Gut 2002; 50: 525–529.
Leonhard-Marek S, Becker G, Breves G et al. Chloride, gluconate, sulfate, and short-chain fatty acids affect calcium flux rates across the sheep forestomach epithelium. J Dairy Sci 2007; 90: 1516–1526.
Uppal SK, Wolf K, Martens H . The effect of short chain fatty acids on calcium flux rates across isolated rumen epithelium of hay-fed and concentrate-fed sheep. J Anim Physiol Anim Nutr (Berl) 2003; 87: 12–20.
Weaver CM . Diet, gut microbiome, and bone health. Curr Osteoporos Rep 2015; 13: 125–130.
Thompson DD, Simmons HA, Pirie CM et al. FDA Guidelines and animal models for osteoporosis. Bone 1995; 17: 125s–133s.
Kurabayashi T, Fujimaki T, Yasuda M et al. Time-course of vertebral and femoral bone loss in rats administered gonadotrophin-releasing hormone agonist. J Endocrinol 1993; 138: 115–125.
Wang Y, Yano T, Kikuchi A et al. Comparison of the effects of add-back therapy with various natural oestrogens on bone metabolism in rats administered a long-acting gonadotrophin-releasing hormone agonist. J Endocrinol 2000; 165: 467–473.
Boyd SK, Davison P, Muller R et al. Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone 2006; 39: 854–862.
Britton RA, Irwin R, Quach D et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol 2014; 229: 1822–1830.
Kimoto-Nira H, Mizumachi K, Okamoto T et al. Influence of long-term consumption of a Lactococcus lactis strain on the intestinal immunity and intestinal flora of the senescence-accelerated mouse. Br J Nutr 2009; 102: 181–185.
Ohlsson C, Engdahl C, Fåk F et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One 2014; 9: e92368.
Parvaneh K, Ebrahimi M, Sabran MR et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int 2015; 2015: 897639.
Klinck J, Boyd SK . The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif Tissue Int 2008; 83: 70–79.
McCabe LR, Irwin R, Schaefer L et al. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol 2013; 228: 1793–1798.
Bouxsein ML, Myers KS, Shultz KL et al. Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res 2005; 20: 1085–1092.
Chiang S-S, Pan T-M . Antiosteoporotic effects of lactobacillus-fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem 2011; 59: 7734–7742.
Li CY, Schaffler MB, Wolde-Semait HT et al. Genetic background influences cortical bone response to ovariectomy. J Bone Miner Res 2005; 20: 2150–2158.
Iwaniec UT, Yuan D, Power RA et al. Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. J Bone Miner Res 2006; 21: 1068–1074.
Legette LL, Lee W-H, Martin BR et al. Genistein, a phytoestrogen, improves total cholesterol, and Synergy, a prebiotic, improves calcium utilization, but there were no synergistic effects. Menopause 2011; 18: 923–931.
Denning TL, Norris BA, Medina-Contreras O et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J Immunol 2011; 187: 733–747.
Pacifici R . Role of T cells in ovariectomy induced bone loss--revisited. J Bone Miner Res 2012; 27: 231–239.
Cenci S, Weitzmann MN, Roggia C et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest 2000; 106: 1229–1237.
Alaish SM, Smith AD, Timmons J et al. Gut microbiota, tight junction protein expression, intestinal resistance, bacterial translocation and mortality following cholestasis depend on the genetic background of the host. Gut Microbes 2013; 4: 292–305.
Davenport ER, Cusanovich DA, Michelini K et al. Genome-wide association studies of the human gut microbiota. PLoS One 2015; 10: e0140301.
Wu HJ, Wu E . The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012; 3: 4–14.
Collins FL, Irwin R, Bierhalter H et al. Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS One 2016; 11: e0153180.
Fuhrman BJ, Feigelson HS, Flores R et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab 2014; 99: 4632–4640.
Flores R, Shi J, Fuhrman B et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med 2012; 10: 253.
Breban M . Gut microbiota and inflammatory joint diseases. Joint Bone Spine 2016; 83: 645–649.
Atarashi K, Tanoue T, Shima T et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011; 331: 337–341.
Nagano Y, Itoh K, Honda K . The induction of Treg cells by gut-indigenous Clostridium. Curr Opin Immunol 2012; 24: 392–397.
Preidis GA, Saulnier DM, Blutt SE et al. Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. FASEB J 2012; 26: 1960–1969.
Schaefer L, Auchtung TA, Hermans KE et al. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 2010; 156: 1589–1599.
Kimoto-Nira H, Suzuki C, Kobayashi M et al. Anti-ageing effect of a lactococcal strain: analysis using senescence-accelerated mice. Br J Nutr 2007; 98: 1178–1186.
Gonzalez-Mariscal L, Tapia R, Chamorro D . Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 2008; 1778: 729–756.
Muto S, Furuse M, Kusano E . Claudins and renal salt transport. Clin Exp Nephrol 2012; 16: 61–67.
Muto S, Hata M, Taniguchi J et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA 2010; 107: 8011–8016.
Yu AS, Enck AH, Lencer WI et al. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 2003; 278: 17350–17359.
Angelow S, Kim KJ, Yu AS . Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. J Physiol 2006; 571: 15–26.
Balda MS, Whitney JA, Flores C et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 1996; 134: 1031–1049.
Ulluwishewa D, Anderson RC, McNabb WC et al. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 2011; 141: 769–776.
van der Helm MW, Odijk M, Frimat JP et al. Direct quantification of transendothelial electrical resistance in organs-on-chips. Biosens Bioelectron 2016; 85: 924–929.
Srinivasan B, Kolli AR, Esch MB et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom 2015; 20: 107–126.
Sumanasekera WK, Zhao L, Ivanova M et al. Effect of estradiol and dihydrotestosterone on hypergravity-induced MAPK signaling and occludin expression in human umbilical vein endothelial cells. Cell Tissue Res 2006; 324: 243–253.
Sumanasekera WK, Sumanasekera GU, Mattingly KA et al. Estradiol and dihydrotestosterone regulate endothelial cell barrier function after hypergravity-induced alterations in MAPK activity. Am J Physiol Cell Physiol 2007; 293: C566–C573.
Ye L, Martin TA, Parr C et al. Biphasic effects of 17-beta-estradiol on expression of occludin and transendothelial resistance and paracellular permeability in human vascular endothelial cells. J Cell Physiol 2003; 196: 362–369.
Hass MA, Nichol P, Lee L et al. Estrogen modulates permeability and prostaglandin levels in the rabbit urinary bladder. Prostaglandins Leukot Essent Fatty Acids 2009; 80: 125–129.
Patrick DM, Leone AK, Shellenberger JJ et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol 2006; 6: 2.
Kinugasa T, Sakaguchi T, Gu X et al. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 2000; 118: 1001–1011.
Anderson RC, Cookson AL, McNabb WC et al. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol 2010; 10: 316.
Qin H, Zhang Z, Hang X et al. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol 2009; 9: 63.
Ewaschuk JB, Diaz H, Meddings L et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 2008; 295: G1025–G1034.
Mennigen R, Nolte K, Rijcken E et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 2009; 296: G1140–G1149.
Peng L, Li ZR, Green RS et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 2009; 139: 1619–1625.
Panigrahi P, Braileanu GT, Chen H et al. Probiotic bacteria change Escherichia coli-induced gene expression in cultured colonocytes: Implications in intestinal pathophysiology. World J Gastroenterol 2007; 13: 6370–6378.
Ardita CS, Mercante JW, Kwon YM et al. Epithelial adhesion mediated by pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses. Appl Environ Microbiol 2014; 80: 5068–5077.
Jones RM, Luo L, Ardita CS et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J 2013; 32: 3017–3028.
Han C, Ding Z, Shi H et al. The role of probiotics in lipopolysaccharide-induced autophagy in intestinal epithelial cells. Cell Physiol Biochem 2016; 38: 2464–2478.
Kim YG, Lee CK, Nah SS et al. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun 2007; 357: 1046–1052.
Zaiss MM, Axmann R, Zwerina J et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum 2007; 56: 4104–4112.
Yuan FL, Li X, Lu WG et al. Regulatory T cells as a potent target for controlling bone loss. Biochem Biophys Res Commun 2010; 402: 173–176.
Luo CY, Wang L, Sun C et al. Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro . Cell Mol Immunol 2011; 8: 50–58.
Sato K, Suematsu A, Okamoto K et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 2006; 203: 2673–2682.
Carlsten H . Immune responses and bone loss: the estrogen connection. Immunol Rev 2005; 208: 194–206.
Nakamura T, Imai Y, Matsumoto T et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 2007; 130: 811–823.
Krum SA, Miranda-Carboni GA, Hauschka PV et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 2008; 27: 535–545.
Martin-Millan M, Almeida M, Ambrogini E et al. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 2010; 24: 323–334.
Manolagas SC, Kousteni S, Jilka RL . Sex steroids and bone. Recent Prog Horm Res 2002; 57: 385–409.
Hughes DE, Dai A, Tiffee JC et al. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med 1996; 2: 1132–1136.
Lelu K, Laffont S, Delpy L et al. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol 2011; 187: 2386–2393.
Chen JR, Plotkin LI, Aguirre JI et al. Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J Biol Chem 2005; 280: 4632–4638.
Wood ZA, Poole LB, Karplus PA . Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003; 300: 650–653.
Ozaki M, Suzuki S, Irani K . Redox factor-1/APE suppresses oxidative stress by inhibiting the rac1 GTPase. FASEB J 2002; 16: 889–890.
Kantengwa S, Jornot L, Devenoges C et al. Superoxide anions induce the maturation of human dendritic cells. Am J Respir Crit Care Med 2003; 167: 431–437.
Grassi F, Tell G, Robbie-Ryan M et al. Oxidative stress causes bone loss in estrogen-deficient mice through enhanced bone marrow dendritic cell activation. Proc Natl Acad Sci USA 2007; 104: 15087–15092.
Lean JM, Davies JT, Fuller K et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 2003; 112: 915–923.
Cenci S, Toraldo G, Weitzmann MN et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA 2003; 100: 10405–10410.
Gao Y, Grassi F, Ryan MR et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest 2007; 117: 122–132.
Ryan MR, Shepherd R, Leavey JK et al. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc Natl Acad Sci USA 2005; 102: 16735–16740.
Yang NN, Venugopalan M, Hardikar S et al. Identification of an estrogen response element activated by metabolites of 17beta-estradiol and raloxifene. Science 1996; 273: 1222–1225.
Adeel S, Singh K, Vydareny KH et al. Bone loss in surgically ovariectomized premenopausal women is associated with T lymphocyte activation and thymic hypertrophy. J Investig Med 2013; 61: 1178–1183.
Ammann P, Rizzoli R, Bonjour JP et al. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J Clin Invest 1997; 99: 1699–1703.
Charatcharoenwitthaya N, Khosla S, Atkinson EJ et al. Effect of blockade of TNF-alpha and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res 2007; 22: 724–729.
D'Amelio P, Grimaldi A, Di Bella S et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 2008; 43: 92–100.
Sherman ML, Weber BL, Datta R et al. Transcriptional and posttranscriptional regulation of macrophage-specific colony stimulating factor gene expression by tumor necrosis factor. Involvement of arachidonic acid metabolites. J Clin Invest 1990; 85: 442–447.
Hofbauer LC, Lacey DL, Dunstan CR et al. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone 1999; 25: 255–259.
Lam J, Takeshita S, Barker JE et al. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000; 106: 1481–1488.
Molnar I, Bohaty I, Somogyine-Vari E . IL-17A-mediated sRANK ligand elevation involved in postmenopausal osteoporosis. Osteoporos Int 2014; 25: 783–786.
Molnar I, Bohaty I, Somogyine-Vari E . High prevalence of increased interleukin-17A serum levels in postmenopausal estrogen deficiency. Menopause 2014; 21: 749–752.
Roberfroid MB, Delzenne NM . Dietary fructans. Annu Rev Nutr 1998; 18: 117–143.
Roberfroid MB, Van Loo JA, Gibson GR . The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr 1998; 128: 11–19.
Cox MA, Jackson J, Stanton M et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol 2009; 15: 5549–5557.
Asarat M, Apostolopoulos V, Vasiljevic T et al. Short-chain fatty acids produced by synbiotic mixtures in skim milk differentially regulate proliferation and cytokine production in peripheral blood mononuclear cells. Int J Food Sci Nutr 2015; 66: 755–765.
Asarat M, Apostolopoulos V, Vasiljevic T et al. Short-chain fatty acids regulate cytokines and Th17/Treg cells in human peripheral blood mononuclear cells in vitro . Immunol Invest 2016; 45: 205–222.
Kwon HK, Lee CG, So JS et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA 2010; 107: 2159–2164.
Lavasani S, Dzhambazov B, Nouri M et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One 2010; 5: e9009.
Thomas CM, Hong T, van Pijkeren JP et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 2012; 7: e31951.
Bouladoux N, Hall JA, Grainger JR et al. Regulatory role of suppressive motifs from commensal DNA. Mucosal Immunol 2012; 5: 623–634.
Agata U, Park JH, Hattori S et al. The effect of different amounts of calcium intake on bone metabolism and arterial calcification in ovariectomized rats. J Nutr Sci Vitaminol (Tokyo) 2013; 59: 29–36.
Arjmandi BH, Hollis BW, Kalu DN . In vivo effect of 17 beta-estradiol on intestinal calcium absorption in rats. Bone Miner 1994; 26: 181–189.
Gallagher JC, Riggs BL, DeLuca HF . Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab 1980; 51: 1359–1364.
Kalu DN, Chen C . Ovariectomized murine model of postmenopausal calcium malabsorption. J Bone Miner Res 1999; 14: 593–601.
O'Loughlin PD, Morris HA . Oestrogen deficiency impairs intestinal calcium absorption in the rat. J Physiol 1998; 511 (Pt 1): 313–322.
Schwartz B, Smirnoff P, Shany S et al. Estrogen controls expression and bioresponse of 1,25-dihydroxyvitamin D receptors in the rat colon. Mol Cell Biochem 2000; 203: 87–93.
Liel Y, Shany S, Smirnoff P et al. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology 1999; 140: 280–285.
Ten Bolscher M, Netelenbos JC, Barto R et al. Estrogen regulation of intestinal calcium absorption in the intact and ovariectomized adult rat. J Bone Miner Res 1999; 14: 1197–1202.
Van Cromphaut SJ, Rummens K, Stockmans I et al. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res 2003; 18: 1725–1736.
Chonan O, Matsumoto K, Watanuki M . Effect of galactooligosaccharides on calcium absorption and preventing bone loss in ovariectomized rats. Biosci Biotechnol Biochem 1995; 59: 236–239.
Zafar TA, Weaver CM, Zhao Y et al. Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. J Nutr 2004; 134: 399–402.
Brennan O, Kuliwaba JS, Lee TC et al. Temporal changes in bone composition, architecture, and strength following estrogen deficiency in osteoporosis. Calcif Tissue Int 2012; 91: 440–449.
Gambacciani M, Levancini M . Management of postmenopausal osteoporosis and the prevention of fractures. Panminerva Med 2014; 56: 115–131.
Gaya P, Medina M, Sánchez-Jiménez A et al. Phytoestrogen metabolism by adult human gut microbiota. Molecules 2016; 21. pii: E1034.
Uehara M . Isoflavone metabolism and bone-sparing effects of daidzein-metabolites. J Clin Biochem Nutr 2013; 52: 193–201.
Ohtomo T, Uehara M, Penalvo JL et al. Comparative activities of daidzein metabolites, equol and O-desmethylangolensin, on bone mineral density and lipid metabolism in ovariectomized mice and in osteoclast cell cultures. Eur J Nutr 2008; 47: 273–279.
Ishimi Y . Dietary equol and bone metabolism in postmenopausal Japanese women and osteoporotic mice. J Nutr 2010; 140: 1373s–1376s.
Tezval M, Sehmisch S, Seidlova-Wuttke D et al. Changes in the histomorphometric and biomechanical properties of the proximal femur of ovariectomized rat after treatment with the phytoestrogens genistein and equol. Planta Med 2010; 76: 235–240.
Legette LL, Prasain J, King J et al. Pharmacokinetics of equol, a soy isoflavone metabolite, changes with the form of equol (dietary versus intestinal production) in ovariectomized rats. J Agric Food Chem 2014; 62: 1294–1300.
Tousen Y, Ezaki J, Fujii Y et al. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: a pilot randomized, placebo-controlled trial. Menopause 2011; 18: 563–574.
Brink E, Coxam V, Robins S et al. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr 2008; 87: 761–770.
Fujioka M, Uehara M, Wu J et al. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J Nutr 2004; 134: 2623–2627.
Wang J, Xu J, Wang B et al. Equol promotes rat osteoblast proliferation and differentiation through activating estrogen receptor. Genet Mol Res 2014; 13: 5055–5063.
Tousen Y, Matsumoto Y, Matsumoto C et al. The combined effects of soya isoflavones and resistant starch on equol production and trabecular bone loss in ovariectomised mice. Br J Nutr 2016; 116: 247–257.
Nishide Y, Tadaishi M, Kobori M et al. Possible role of S-equol on bone loss via amelioration of inflammatory indices in ovariectomized mice. J Clin Biochem Nutr 2013; 53: 41–48.
Kang JS, Yoon YD, Han MH et al. Estrogen receptor-independent inhibition of tumor necrosis factor-alpha gene expression by phytoestrogen equol is mediated by blocking nuclear factor-kappaB activation in mouse macrophages. Biochem Pharmacol 2005; 71: 136–143.
Guadamuro L, Dohrmann AB, Tebbe CC et al. Bacterial communities and metabolic activity of faecal cultures from equol producer and non-producer menopausal women under treatment with soy isoflavones. BMC Microbiol 2017; 17: 93.
Possemiers S, Bolca S, Eeckhaut E et al. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: producer phenotyping and relation with intestinal community. FEMS Microbiol Ecol 2007; 61: 372–383.
Bolca S, Wyns C, Possemiers S et al. Cosupplementation of isoflavones, prenylflavonoids, and lignans alters human exposure to phytoestrogen-derived 17beta-estradiol equivalents. J Nutr 2009; 139: 2293–2300.
Yu ZT, Yao W, Zhu WY . Isolation and identification of equol-producing bacterial strains from cultures of pig faeces. FEMS Microbiol Lett 2008; 282: 73–80.
Nakatsu CH, Armstrong A, Clavijo AP et al. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS One 2014; 9: e108924.
Decroos K, Vanhemmens S, Cattoir S et al. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol 2005; 183: 45–55.
Fujii S, Takahashi N, Inoue H et al. A combination of soy isoflavones and cello-oligosaccharides changes equol/O-desmethylangolensin production ratio and attenuates bone fragility in ovariectomized mice. Biosci Biotechnol Biochem 2016; 80: 1632–1635.
Ohta A, Uehara M, Sakai K et al. A combination of dietary fructooligosaccharides and isoflavone conjugates increases femoral bone mineral density and equol production in ovariectomized mice. J Nutr 2002; 132: 2048–2054.
Mathey J, Mardon J, Fokialakis N et al. Modulation of soy isoflavones bioavailability and subsequent effects on bone health in ovariectomized rats: the case for equol. Osteoporos Int 2007; 18: 671–679.
Devareddy L, Khalil DA, Korlagunta K et al. The effects of fructo-oligosaccharides in combination with soy protein on bone in osteopenic ovariectomized rats. Menopause 2006; 13: 692–699.
Wu J, Oka J, Ezaki J et al. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: a double-blind, randomized, controlled trial. Menopause 2007; 14: 866–874.
Hoy-Schulz YE, Jannat K, Roberts T et al. Safety and acceptability of Lactobacillus reuteri DSM 17938 and Bifidobacterium longum subspecies infantis 35624 in Bangladeshi infants: a phase I randomized clinical trial. BMC Complement Altern Med 2016; 16: 44.
Kitajima H, Hirano S . Safety of Bifidobacterium breve (BBG-01) in preterm infants. Pediatr Int 2017; 59: 328–333.
Ladas EJ, Bhatia M, Chen L et al. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant 2016; 51: 262–266.
Besselink MG, van Santvoort HC, Buskens E et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371: 651–659.
Acknowledgements
We thank Dr. Geelsu Hwang from the University of Pennsylvania School of Dental Medicine for critical advice on the manuscript. This work was supported by the National Natural Science Foundation of China (grant numbers 81430011, 81470711, and 81670978) and the Brilliant Young Investigator Award, Sichuan University (grant number 2015SCU04A16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xu, X., Jia, X., Mo, L. et al. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res 5, 17046 (2017). https://doi.org/10.1038/boneres.2017.46
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/boneres.2017.46
This article is cited by
-
Impact of metabolic syndrome on bone mineral density in men over 50 and postmenopausal women according to U.S. survey results
Scientific Reports (2024)
-
The role and applications of extracellular vesicles in osteoporosis
Bone Research (2024)
-
Mechanistic study on the alleviation of postmenopausal osteoporosis by Lactobacillus acidophilus through butyrate-mediated inhibition of osteoclast activity
Scientific Reports (2024)
-
The potential of Klebsiella and Escherichia-Shigella and amino acids metabolism to monitor patients with postmenopausal osteoporosis in northwest China
BMC Microbiology (2023)
-
Gold-nanosphere mitigates osteoporosis through regulating TMAO metabolism in a gut microbiota-dependent manner
Journal of Nanobiotechnology (2023)