Abstract
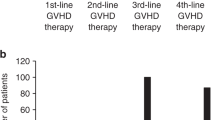
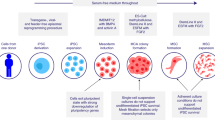
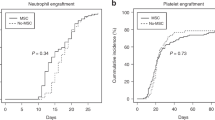
Acute GvHD (aGvHD) is a life-threatening complication of hematopoietic stem cell transplantation. Frontline therapy for aGvHD consists of corticosteroid administration. However, ∼25% of the patients have a steroid-refractory disease, a sign of poor prognosis. An alternative therapy for steroid-refractory aGvHD is infusion of mesenchymal stromal cells (MSCs). Herein, we report the results of 46 patients treated with MSC infusion as salvage therapy for steroid-refractory aGvHD III/IV (78% grade IV). Patients received a median cumulative dose of MSCs of 6.81 × 106/kg (range, 0.98–29.78 × 106/kg) in a median of 3 infusions (range, 1–7). Median time between the onset of aGvHD and the first MSC infusion was 25.5 days (range, 6–153). Of the patients, 50% (23/46) presented clinical improvement. Of these, 3 patients (13%) had complete response, 14 (61%) had partial response and 6 (26%) had transient partial response. The estimated probability of survival at 2s year was 17.4%. Only 2 patients (4.3%) presented acute transient side effects (nausea/vomiting and blurred vision) during cell infusion. No patient had late or severe side effects because of MSC infusion. These results suggest that this therapeutic modality is safe and should be considered for steroid-refractory aGvHD, especially in countries where other second-line agents are less available.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Jagasia M, Arora M, Flowers MED, Chao NJ, McCarthy PL, Cutler CS et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012; 119: 296–307.
Ferrara JL, Levine JE, Reddy P, Holler E . Graft-versus-host disease. Lancet 2009; 373: 1550–1561.
Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol 2012; 158: 30–45.
Koreth J, Antin JH . Current and future approaches for control of graft-versus-host disease. Expert Rev Hematol 2008; 1: 111.
Alousi AM, Weisdorf DJ, Logan BR, Bolaños-Meade J, Carter S, Difronzo N et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood 2009; 114: 511–517.
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371: 1579–1586.
Xhaard A, Rocha V, Bueno B, de Latour RP, Lenglet J, Petropoulou A et al. Steroid-refractory acute GVHD: lack of long-term improved survival using new generation anticytokine treatment. Biol Blood Marrow Transplant 2012; 18: 406–413.
Deeg HJ . How I treat how I treat refractory acute GVHD. Blood 2007; 109: 4119–4126.
Ho VT, Cutler C . Current prevention and therapies in acute GVHD. Best Pract Res Clin Haematol 2008; 21: 223–237.
Garnett C, Apperley JF, Pavlů J . Treatment and management of graft-versus-host disease: improving response and survival. Ther Adv Hematol 2013; 4: 366–378.
Najar M, Raicevic G, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L . Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy 2016; 18: 160–171.
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M et al. Treatment of severe acute graft-versus-host disease with third party haplindentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441.
Kebriaei P, Robinson S . Treatment of graft-versus-host-disease with mesenchymal stromal cells. Cytotherapy 2011; 13: 262–268.
von Bahr L, Sundberg B, Lönnies L, Sander B, Karbach H, Hägglund H et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant 2012; 18: 557–564.
Ball LM, Bernardo ME, Roelofs H, van Tol MJD, Contoli B, Zwaginga JJ et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III–IV acute graft-versus-host disease. Br J Haematol 2013; 163: 501–509.
Resnick IB, Barkats C, Shapira MY, Stepensky P, Bloom AI, Shimoni A et al. Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC). Am J Blood Res 2013; 3: 225–238.
Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW et al. Allogeneic human mesenchymal stem cell therapy (Remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant 2014; 20: 229–235.
Herrmann RP, Sturm MJ . Adult human mesenchymal stromal cells and the treatment of graft versus host disease. Stem Cells Cloning 2014; 7: 45–52.
Introna M, Lucchini G, Dander E, Galimberti S, Rovelli A, Balduzzi A et al. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant 2014; 20: 375–381.
Sánchez-Guijo F, Caballero-Velázquez T, López-Villar O, Redondo A, Parody R, Martínez C et al. Sequential third-party mesenchymal stromal cell therapy for refractory acute graft-versus-host disease. Biol Blood Marrow Transplant 2014; 20: 1580–1585.
Muroi K, Miyamura K, Okada M, Yamashita T, Murata M, Ishikawa T et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol 2016; 103: 243–250.
von Dalowski F, Kramer M, Wermke M, Wehner R, Rollig C, Alakel N et al. Mesenchymal stromal cells for treatment of acute steroid-refractory graft versus host disease: clinical responses and long-term outcome. Stem Cells 2016; 34: 357–366.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317.
Caruso SR, Orellana MD, Mizukami A, Fernandes TR, Fontes AM, Suazo CAT et al. Growth and functional harvesting of human mesenchymal stromal cells cultured on a microcarrier-based system. Biotechnol Prog 2014; 30: 889–895.
Kaipe H, Erkers T, Sadeghi B, Ringdén O . Stromal cells–are they really useful for GVHD? Bone Marrow Transplant 2014; 49: 737–743.
Hashmi S, Ahmed M, Murad MH, Litzow MR, Adams RH, Ball LM et al. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol 2016; 3: e45–e52.
Acknowledgements
We thank Patrícia A Tozetti, Patrícia VB Palma and Camila COM Bonaldo for technical assistance. This work received financial support from FINEP (01.08.0591.03); CNPq (310619/2012-2); and Center for Cell-based Therapy–São Paulo Research Foundation FAPESP (CEPID 13/08135-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dotoli, G., De Santis, G., Orellana, M. et al. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant 52, 859–862 (2017). https://doi.org/10.1038/bmt.2017.35
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.35
This article is cited by
-
A review of the application of mesenchymal stem cells in the field of hematopoietic stem cell transplantation
European Journal of Medical Research (2023)
-
Evaluation of safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells in pediatric bronchiolitis obliterans syndrome (BoS) after allogeneic hematopoietic stem cell transplantation (allo-HSCT)
Stem Cell Research & Therapy (2023)
-
Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy
Journal of Biological Engineering (2023)
-
Current perspectives on mesenchymal stromal cell therapy for graft versus host disease
Cellular & Molecular Immunology (2023)
-
Understanding and treatment of cutaneous graft-versus-host-disease
Bone Marrow Transplantation (2023)