Abstract
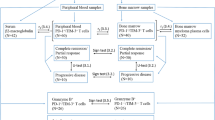
The proportion of multiple myeloma patients in long-term complete response (LTCR-MM) for more than 6 years after autologous stem cell transplantation (ASCT) is small. To evaluate whether this LTCR is associated with a particular immune signature, peripheral blood samples from 13 LTCR-MM after ASCT and healthy blood donors (HBD) were analysed. Subpopulations of T-cells (naïve, effector, central memory and regulatory), B-cells (naïve, marginal zone-like, class-switched memory, transitional and plasmablasts) and NK-cells expressing inhibitory and activating receptors were quantified by multiparametric flow cytometry (MFC). Heavy/light chains (HLC) were quantified by nephelometry. The percentage of CD4+ T-cells was lower in patients, whereas an increment in the percentage of CD4+ and CD8+ effector memory T-cells was associated with the LTCR. Regulatory T-cells and NK-cells were similar in both groups but a particular redistribution of inhibitory and activating receptors in NK-cells were found in patients. Regarding B-cells, an increase in naïve cells and a corresponding reduction in marginal zone-like and class-switched memory B-cells was observed. The HLC values were normal. Our results suggest that LTCR-MM patients express a particular immune signature, which probably reflects a ‘high quality’ immune reconstitution that could exert a competent anti-tumor immunological surveillance along with a recovery of the humoral immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gay F, Larocca A, Wijermans P, Cavallo F, Rossi D, Schaafsma R et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood 2011; 117: 3025–3031.
Ocio EM, Richardson PG, Rajkumar SV, Palumbo A, Mateos MV, Orlowski R et al. New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia 2014; 28: 525–542.
Kyle RA, Rajkumar SV . An overview of the progress in the treatment of multiple myeloma. Expert Rev Hematol 2014; 7: 5–7.
Moreau P, Attal M, Facon T . Frontline therapy of multiple myeloma. Blood 2015; 125: 3076–3084.
Turesson I, Velez R, Kristinsson SY, Landgren O . Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol 2010; 28: 830–834.
Ludwig H, Bolejack V, Crowley J, Bladé J, Miguel JS, Kyle RA et al. Survival and years of life lost in different age cohorts of patients with multiple myeloma. J Clin Oncol 2010; 28: 1599–1605.
Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 2016; 389: 519–527.
Wang M, Delasalle K, Feng L, Thomas S, Giralt S, Qazilbash M et al. CR represents an early index of potential long survival in multiple myeloma. Bone Marrow Transplant. 2010; 45: 498–504.
Martinez-Lopez J, Blade J, Mateos M-V, Grande C, Alegre A, García-Laraña J et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood 2011; 118: 529–534.
Paiva B, Gutiérrez NC, Rosiñol L, Vídriales M-B, Montalbán M-Á, Martínez-López J et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood 2012; 119: 687–691.
Kumar L, Boya RR, Pai R, Harish P, Mookerjee A, Sainath B et al. Autologous stem cell transplantation for multiple myeloma: long-term results. Natl Med J India 29: 192–199.
Rajkumar SV . Treatment of myeloma: cure vs control. Mayo Clin Proc 2008; 83: 1142–1145.
Kumar SK, Rajkumar SV . The current status of minimal residual disease assessment in myeloma. Leukemia 2014; 28: 239–240.
Martinez-Lopez J, Lahuerta JJ, Pepin F, González M, Barrio S, Ayala R et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood 2014; 123: 3073–3079.
Rawstron AC, Gregory WM, de Tute RM, Davies FE, Bell SE, Drayson MT et al. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood 2015; 125: 1932–1935.
Sherrod AM, Hari P, Mosse CA, Walker RC, Cornell RF . Minimal residual disease testing after stem cell transplantation for multiple myeloma. Bone Marrow Transplant. 2015; 51: 2–12.
Paiva B, Puig N, Garcia-Sanz R, San Miguel JF . Is This the time to introduce minimal residual disease in multiple myeloma clinical practice? Clin Cancer Res 2015; 21: 2001–2008.
Ege H, Gertz MA, Svetomir N, Lacy MQ, Hayman SR, Kumar K et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol 2008; 141: 792–798.
Jimenez-zepeda VH, Reece DE, Trudel S, Chen C, Franke N, Winter A et al. Absolute lymphocyte count as predictor of overall survival for patients with multiple myeloma treated with single autologous stem cell transplant. Leuk Lymphoma 2015; 56: 2668–2673.
Dasanu CA . Immune alterations in untreated and treated multiple myeloma. J Oncol Pharm Pract 2012; 18: 257–263.
Dosani T, Carlsten M, Maric I, Landgren O . The cellular immune system in myelomagenesis: NK cells and T cells in the development of MM and their uses in immunotherapies. Blood Cancer J 2015; 5: e306.
Raitakari M, Brown RD, Sze D, Yuen E, Barrow L, Nelson M et al. T-cell expansions in patients with multiple myeloma have a phenotype of cytotoxic T cells. Br J Haematol 2000; 110: 203–209.
Pérez-Andres M, Almeida J, Martin-Ayuso M, Moro MJ, Martin-Nuñez G, Galende J et al. Characterization of bone marrow T cells in monoclonal gammopathy of undetermined significance, multiple myeloma, and plasma cell leukemia demonstrates increased infiltration by cytotoxic/Th1 T cells demonstrating a squed TCR-Vbeta repertoire. Cancer 2006; 106: 1296–1305.
Muthu Raja KR, Rihova L, Zahradova L, Klincova M, Penka M, Hajek R . Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS ONE 2012; 7: e47077.
Feyler S, von Lilienfeld-Toal M, Jarmin S, Marles L, Rawstron A, Ashcroft AJ et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells are increased whilst CD3(+)CD4(-)CD8(-)alphabetaTCR(+) double negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br J Haematol 2009; 144: 686–695.
Ludwig H, Milosavljevic D, Zojer N, Faint JM, Bradwell AR, Hübl W et al. Immunoglobulin heavy/light chain ratios improve paraprotein detection and monitoring, identify residual disease and correlate with survival in multiple myeloma patients. Leukemia 2013; 27: 213–219.
Katzmann Ja, Clark R, Kyle Ra, Larson DR, Therneau TM, Melton LJ et al. Suppression of uninvolved immunoglobulins defined by heavy/light chain pair suppression is a risk factor for progression of MGUS. Leukemia 2013; 27: 208–212.
Koulieris E, Panayiotidis P, Harding SJ, Kafasi N, Maltezas D, Bartzis V et al. Ratio of involved/uninvolved immunoglobulin quantification by Hevylite assay: clinical and prognostic impact in multiple myeloma. Exp Hematol Oncol 2012; 1: 9.
Stetler-Stevenson M, Paiva B, Stoolman L, Lin P, Jorgensen JL, Orfao A et al. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytometry B Clin Cytom 2015; 90: 26–30.
Pérez-Persona E, Vidriales M-B, Mateo G, García-Sanz R, Mateos M-V, de Coca AG et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007; 110: 2586–2592.
Durie BGM, Harousseau J-L, Miguel JS, Bladé J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473.
Ludwig H, Miguel JS, Dimopoulos MA, Palumbo A, Garcia Sanz R, Powles R et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia 2014; 28: 981–992.
Pessoa de Magalhães RJ, Vidriales M-B, Paiva B, Fernandez-Gimenez C, García-Sanz R, Mateos M-V et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica 2013; 98: 79–86.
Chung DJ, Pronschinske KB, Shyer JA, Sharma S, Leung S, Curran SA et al. T-cell exhaustion in multiple myeloma relapse after autotransplant: optimal timing of immunotherapy. Cancer Immunol Res 2016; 4: 61–71.
Rosenblatt J, Avigan D . Targeting the PD-1/PD-L1 axis in multiple myeloma: a dream or a reality. Blood 2016; 129: 275–279.
Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A . Surface NK receptors and their ligands on tumor cells. Semin Immunol 2006; 18: 151–158.
Jurisic V, Srdic T, Konjevic G, Markovic O, Colovic M . Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol 2007; 24: 312–317.
El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007; 67: 8444–8449.
Meehan KR, Talebian L, Tosteson TD, Hill JM, Szczepiorkowski Z, Sentman CL et al. Adoptive cellular therapy using cells enriched for NKG2D+CD3+CD8+T cells after autologous transplantation for myeloma. Biol Blood Marrow Transplant. 2013; 19: 129–137.
Morice WG . The immunophenotypic attributes of NK cells and NK-cell lineage lymphoproliferative disorders. Am J Clin Pathol 2007; 127: 881–886.
Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F, Cutting Edge . NKG2D Is a costimulatory receptor for human naive CD8+ T cells. J Immunol 2005; 174: 4480–4484.
Talebian L, Fischer DA, Wu J, Channon JY, Sentman CL, Ernstoff MS et al. The natural killer-activating receptor, NKG2D, on CD3+CD8+ T cells plays a critical role in identifying and killing autologous myeloma cells. Transfusion 2014; 54: 1515–1521.
Tovar N, Fernández de Larrea C, Elena M, Cibeira MT, Aróstegui JI, Rosiñol L et al. Prognostic impact of serum immunoglobulin heavy/light chain ratio in patients with multiple myeloma in complete remission after autologous stem cell transplantation. Biol Blood Marrow Transpl 2012; 18: 1076–1079.
Kocoglu M, Badros A . The role of immunotherapy in multiple myeloma. Pharmaceuticals 2016; 9: 3.
Lonial S, Vij R, Harousseau J-L, Facon T, Moreau P, Mazumder A et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 2012; 30: 1953–1959.
van der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PWHI et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica 2011; 96: 284–290.
Paiva B, Cedena M-T, Puig N, Arana P, Vidriales M-B, Cordon L et al. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood 2016; 127: 3165–3174.
Acknowledgements
We thank all patients and clinical staff who made this study possible. We are very grateful to Ana Ramírez and Víctor López for their valuable help with flow cytometry, to Lorena Vega for statistics advice and to Lawrence Baron for his help with the writing. The grants include Grant PI12/00494P and PI15/02085 from the Fondo de Investigaciones Sanitarias to CMC supported this work. CMC was co-financed by FEDER funds. AAL is supported by a research grant from the JL Castaño Foundation since September 2015. BA and MGP were granted for the Spanish Leukemia and Lyphoma Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AA is a member of Advisory Boards of Janssen-Cilag, Celgene and Amgen. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Arteche-López, A., Kreutzman, A., Alegre, A. et al. Multiple myeloma patients in long-term complete response after autologous stem cell transplantation express a particular immune signature with potential prognostic implication. Bone Marrow Transplant 52, 832–838 (2017). https://doi.org/10.1038/bmt.2017.29
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.29
This article is cited by
-
The immunome of mobilized peripheral blood stem cells is predictive of long-term outcomes and therapy-related myeloid neoplasms in patients with multiple myeloma undergoing autologous stem cell transplant
Blood Cancer Journal (2023)
-
The metabolic profile of reconstituting T-cells, NK-cells, and monocytes following autologous stem cell transplantation and its impact on outcome
Scientific Reports (2022)
-
Deepening responses associated with improved progression-free survival with ixazomib versus placebo as posttransplant maintenance in multiple myeloma
Leukemia (2020)
-
The timing of plerixafor addition to G-Csf and chemotherapy affects immunological recovery after autologous stem cell transplant in multiple myeloma
Bone Marrow Transplantation (2020)
-
Characteristics of exceptional responders to autologous stem cell transplantation in multiple myeloma
Blood Cancer Journal (2020)