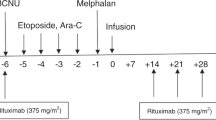
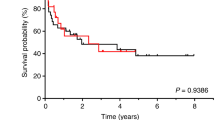
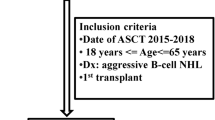
Abstract
High-dose chemotherapy preceding autologous hematopoietic stem cell transplantation (auto-HSCT) is one treatment option for patients with Hodgkin (HL) or non-Hodgkin lymphoma (NHL). The most frequently used intensive chemotherapy is a combination of carmustine (BCNU), etoposide, cytarabine and melphalan (BEAM). However, BCNU is consistently in short supply, and there has been a recent dramatic increase in its cost, necessitating the utilization of conditioning alternatives. The busulfan-based conditioning regimen known as the busulfan–cyclophosphamide–etoposide (BuCyE) combination is the second most-studied conditioning regimen worldwide after BEAM, and it exhibits a benefit/risk ratio that is comparable to that of BEAM. In addition to these two combinations, the present manuscript also summarizes data reported for other conditioning combinations. Owing to the lack of prospective and comparative studies, a comparison of the toxicities and medicoeconomical profiles of these treatments is warranted to identify effective replacements for BCNU-based conditioning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rancea M, Will A, Borchmann P, Monsef I, Engert A, Skoetz N . Sixteenth biannual report of the Cochrane Haematological Malignancies Group: focus on Non-Hodgkin's lymphoma. J Natl Cancer Inst 2014; 106: 1–11.
Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant 2015; 50: 476–482.
Tilly H, Vitolo U, Walewski J, da Silva MG, Shpilberg O, Andre M et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23 (Suppl 7): vii78–vii82.
Gunnellini M, Emili R, Coaccioli S, Liberati AM . The role of autologous stem cell transplantation in the treatment of diffuse large B-cell lymphoma. Adv Hematol 2012; 2012: 195484.
Dreyling M, Geisler C, Hermine O, Kluin-Nelemans HC, Le Gouill S, Rule S et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25 (Suppl 3): iii83–iii92.
Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, Ladetto M . Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25 (Suppl 3): iii76–iii82.
Rohatiner AZ, Nadler L, Davies AJ, Apostolidis J, Neuberg D, Matthews J et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: long-term follow-up. J Clin Oncol 2007; 25: 2554–2559.
Le Gouill S, De Guibert S, Planche L, Brice P, Dupuis J, Cartron G et al. Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study. Haematologica 2011; 96: 1128–1135.
Giulia P, Corradini P . Autologous stem cell transplantation for T-cell lymphomas. Semin Hematol 2014; 51: 59–66.
d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol 2012; 30: 3093–3099.
Friedberg JW, Neuberg D, Stone RM, Alyea E, Jallow H, LaCasce A et al. Outcome in patients with myelodysplastic syndrome after autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 1999; 17: 3128–3135.
Fernandez HF, Escalon MP, Pereira D, Lazarus HM . Autotransplant conditioning regimens for aggressive lymphoma: are we on the right road? Bone Marrow Transplant 2007; 40: 505–513.
Puig N, de la Rubia J, Remigia MJ, Jarque I, Martin G, Cupelli L et al. Morbidity and transplant-related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem-cell transplantation. Leuk Lymphoma 2006; 47: 1488–1494.
Sharma A, Kayal S, Iqbal S, Malik PS, Raina V . Comparison of BEAM vs LEAM regimen in autologous transplant for lymphoma at AIIMS. Springerplus 2013; 2: 489.
Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res 2008; 14: 309–317.
Darwish M, Bond M, Hellriegel E, Robertson P Jr, Chovan JP . Pharmacokinetic and pharmacodynamic profile of bendamustine and its metabolites. Cancer Chemother Pharmacol 2015; 75: 1143–1154.
Dubbelman AC, Rosing H, Darwish M, D'Andrea D, Bond M, Hellriegel E et al. Pharmacokinetics and excretion of 14C-bendamustine in patients with relapsed or refractory malignancy. Drugs R D 13: 17–28.
Strumberg D, Harstrick A, Doll K, Hoffmann B, Seeber S . Bendamustine hydrochloride activity against doxorubicin-resistant human breast carcinoma cell lines. Anticancer Drugs 1996; 7: 415–421.
Bremer K . High rates of long-lasting remissions after 5-day bendamustine chemotherapy cycles in pre-treated low-grade non-Hodgkin's-lymphomas. J Cancer Res Clin Oncol 2002; 128: 603–609.
Cheson BD, Wendtner CM, Pieper A, Dreyling M, Friedberg J, Hoelzer D et al. Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk 10: 21–27.
Bergmann MA, Goebeler ME, Herold M, Emmerich B, Wilhelm M, Ruelfs C et al. Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group. Haematologica 2005; 90: 1357–1364.
Lissitchkov T, Arnaudov G, Peytchev D, Merkle K . Phase-I/II study to evaluate dose limiting toxicity, maximum tolerated dose, and tolerability of bendamustine HCl in pre-treated patients with B-chronic lymphocytic leukaemia (Binet stages B and C) requiring therapy. J Cancer Res Clin Oncol 2006; 132: 99–104.
Owen JS, Melhem M, Passarell JA, D'Andrea D, Darwish M, Kahl B . Bendamustine pharmacokinetic profile and exposure-response relationships in patients with indolent non-Hodgkin's lymphoma. Cancer Chemother Pharmacol 2010; 66: 1039–1049.
Rasschaert M, Schrijvers D, Van den Brande J, Dyck J, Bosmans J, Merkle K et al. A phase I study of bendamustine hydrochloride administered day 1+2 every 3 weeks in patients with solid tumours. Br J Cancer 2007; 96: 1692–1698.
Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol 2005; 23: 3383–3389.
Friedberg JW, Cohen P, Chen L, Robinson KS, Forero-Torres A, La Casce AS et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin's lymphoma: results from a phase II multicenter, single-agent study. J Clin Oncol 2008; 26: 204–210.
Visco C, Finotto S, Zambello R, Paolini R, Menin A, Zanotti R et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol 2013; 31: 1442–1449.
Ohmachi K, Ando K, Ogura M, Uchida T, Itoh K, Kubota N et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci 2010; 101: 2059–2064.
Herbaux C, Genet P, Bouabdallah K, Pignon JM, Debarri H, Guidez S et al. Bendamustine is effective in T-Cell prolymphocytic leukaemia. Br J Haematol 2015; 168: 916–919.
Damaj G, Gressin R, Bouabdallah K, Cartron G, Choufi B, Gyan E et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol 2013; 31: 104–110.
Moskowitz AJ, Hamlin PA Jr, Perales MA, Gerecitano J, Horwitz SM, Matasar MJ et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol 2013; 31: 456–460.
Ghesquieres H, Stamatoullas A, Casasnovas O, Morschhauser F, Gyan E, Gabarre J et al. Clinical experience of bendamustine in relapsed or refractory Hodgkin lymphoma: a retrospective analysis of the French compassionate use program in 28 patients. Leuk Lymphoma 2013; 54: 2399–2404.
Corazzelli G, Angrilli F, D'Arco A, Ferrara F, Musto P, Guarini A et al. Efficacy and safety of bendamustine for the treatment of patients with recurring Hodgkin lymphoma. Br J Haematol 2013; 160: 207–215.
Damaj G, Malard F, Hulin C, Caillot D, Garidi R, Royer B et al. Efficacy of bendamustine in relapsed/refractory myeloma patients: results from the French compassionate use program. Leuk Lymphoma 2012; 53: 632–634.
Knop S, Straka C, Haen M, Schwedes R, Hebart H, Einsele H . The efficacy and toxicity of bendamustine in recurrent multiple myeloma after high-dose chemotherapy. Haematologica 2005; 90: 1287–1288.
Ponisch W, Rozanski M, Goldschmidt H, Hoffmann FA, Boldt T, Schwarzer A et al. Combined bendamustine, prednisolone and thalidomide for refractory or relapsed multiple myeloma after autologous stem-cell transplantation or conventional chemotherapy: results of a Phase I clinical trial. Br J Haematol 2008; 143: 191–200.
Darwish M, Megason G, Bond M, Hellriegel E, Robertson P Jr, Grasela T et al. Population pharmacokinetics and pharmacokinetics/pharmacodynamics of bendamustine in pediatric patients with relapsed/refractory acute leukemia. Curr Med Res Opin 2014; 30: 2305–2315.
Cheson BD, Brugger W, Damaj G, Dreyling M, Kahl B, Kimby E et al. Optimal use of bendamustine in hematologic disorders: treatment recommendations from an international consensus panel - an update. Leuk Lymphoma 2016; 57: 766–782.
Visani G, Malerba L, Stefani PM, Capria S, Galieni P, Gaudio F et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood 2011; 118: 3419–3425.
Visani G, Stefani PM, Capria S, Malerba L, Galieni P, Gaudio F et al. Bendamustine, etoposide, cytarabine, melphalan, and autologous stem cell rescue produce a 72% 3-year PFS in resistant lymphoma. Blood 2014; 124: 3029–3031.
Noesslinger T, Moesti M, Tinchon C, Koller E, Linkesch W, Keil F . Autologous stem cell transplantation with Benda-BEAM (bendamustine, etoposide, cytarabine, melphalan) in aggressive non Hodgkin and Hodgkin's lymphoma. Blood 2014; 124: 2515.
Martino M, Messina G, Moscato T, Fedele R, Console G, Console G et al. Bendamustine plus melphalan as conditioning regimen for second autologous stem cell transplantation in patients with multiple myeloma: single centre experience. Blood 2014; 124: 2516.
Martín A, Redondo AM, Valcárcel D, Gonzalez AP, Guillermo Al, Bello JL et al. A phase 2 study from Spanish Geltamo Group investigating the efficacy and safety of bendamustine as part of conditioning regimen for autologous stem-cell transplantation in patients with aggressive lymphomas: Second Interim Analysis. Blood 2014; 124: 2524.
Garciaz S, Coso D, Schiano de Collela JM, Broussais F, Stoppa AM, Aurran T et al. Bendamustine-based conditioning for non-Hodgkin lymphoma autologous transplantation: an increasing risk of renal toxicity. Bone Marrow Transplant 2016; 51: 319–321.
Bains T, Chen AI, Lemieux A, Hayes-Lattin BM, Leis JF, Dibb W et al. Improved outcome with busulfan, melphalan and thiotepa conditioning in autologous hematopoietic stem cell transplant for relapsed/refractory Hodgkin lymphoma. Leuk Lymphoma 2014; 55: 583–587.
Di Ianni M, Ballanti S, Iodice G, Reale A, Falzetti F, Minelli O et al. High-dose thiotepa, etoposide and carboplatin as conditioning regimen for autologous stem cell transplantation in patients with high-risk Hodgkin's lymphoma. Hematology 2012; 17: 23–27.
Gutierrez-Delgado F, Holmberg L, Hooper H, Petersdorf S, Press O, Maziarz R et al. Autologous stem cell transplantation for Hodgkin's disease: busulfan, melphalan and thiotepa compared to a radiation-based regimen. Bone Marrow Transplant 2003; 32: 279–285.
Wadehra N, Farag S, Bolwell B, Elder P, Penza S, Kalaycio M et al. Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation. Biol Blood Marrow Transplant 2006; 12: 1343–1349.
Gopal AK, Gooley TA, Golden JB, Maloney DG, Bensinger WI, Petersdorf SH et al. Efficacy of high-dose therapy and autologous hematopoietic stem cell transplantation for non-Hodgkin's lymphoma in adults 60 years of age and older. Bone Marrow Transplant 2001; 27: 593–599.
Gutierrez-Delgado F, Maloney DG, Press OW, Golden J, Holmberg LA, Maziarz RT et al. Autologous stem cell transplantation for non-Hodgkin's lymphoma: comparison of radiation-based and chemotherapy-only preparative regimens. Bone Marrow Transplant 2001; 28: 455–461.
Lee SC, Kim SJ, Lee DH, Kim WS, Suh C, Won JH . Excessive toxicity of once daily i.v. BU, melphalan and thiotepa followed by auto SCT on patients with non-Hodgkin's lymphoma. Bone Marrow Transplant 2010; 45: 801–802.
Anagnostopoulos A, Aleman A, Ayers G, Donato M, Champlin R, Weber D et al. Comparison of high-dose melphalan with a more intensive regimen of thiotepa, busulfan, and cyclophosphamide for patients with multiple myeloma. Cancer 2004; 100: 2607–2612.
Shimoni A, Smith TL, Aleman A, Weber D, Dimopoulos M, Anderlini P et al. Thiotepa, busulfan, cyclophosphamide (TBC) and autologous hematopoietic transplantation: an intensive regimen for the treatment of multiple myeloma. Bone Marrow Transplant 2001; 27: 821–828.
Dimopoulos MA, Alexanian R, Przepiorka D, Hester J, Andersson B, Giralt S et al. Thiotepa, busulfan, and cyclophosphamide: a new preparative regimen for autologous marrow or blood stem cell transplantation in high-risk multiple myeloma. Blood 1993; 82: 2324–2328.
Heideman RL, Cole DE, Balis F, Sato J, Reaman GH, Packer RJ et al. Phase I and pharmacokinetic evaluation of thiotepa in the cerebrospinal fluid and plasma of pediatric patients: evidence for dose-dependent plasma clearance of thiotepa. Cancer Res 1989; 49: 736–741.
Hassan M, Ehrsson H, Smedmyr B, Totterman T, Wallin I, Oberg G et al. Cerebrospinal fluid and plasma concentrations of busulfan during high-dose therapy. Bone Marrow Transplant 1989; 4: 113–114.
Oh DH, Chua N, Street L, Stewart DA . Treatment of patients with secondary central nervous system lymphoma with high-dose busulfan/thiotepa-based conditioning and autologous stem cell transplant. Leuk Lymphoma 2015; 57: 1–6.
Zaucha R, Gooley T, Holmberg L, Gopal AK, Press O, Maloney D et al. High-dose chemotherapy with BEAM or busulphan/melphalan and thiotepa followed by hematopoietic cell transplantation in malignant lymphoma. Leuk Lymphoma 2008; 49: 1899–1906.
Sellner L, Boumendil A, Finel H, Choquet S, de Rosa G, Falzetti F et al. Thiotepa-based high-dose therapy for autologous stem cell transplantation in lymphoma: a retrospective study from the EBMT. Bone Marrow Transplant 2016; 51: 212–218.
Carella AM, Palumbo G, Merla E, Greco MM, Fontana A, Specchia G et al. TEAM (thiotepa, etoposide, cytarabine, melphalan) as conditioning regimen for lymphoma treatment with autologous haematopoietic stem cell transplantation. Bone Marrow Transplantation 2014; 49 (Suppl 1): S177 (abstract P161).
Illerhaus G, Marks R, Ihorst G, Guttenberger R, Ostertag C, Derigs G et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol 2006; 24: 3865–3870.
Illerhaus G, Muller F, Feuerhake F, Schafer AO, Ostertag C, Finke J . High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica 2008; 93: 147–148.
Cumpston AD, Bulion DA, Kamate A, Weisenborn R, Bunner P, Visweshwar N et al. Outcome with a thiotepa containing regimen and autologous HSCT in patients with NHL. Biol Blood Marrow Transplant 2007; 13: 37.
Shah NA, Rauenzahn S, Wen S, Craig M, Kanate AS, Hamadani M et al. Long term outcomes of autologous hematopoietic cell transplant (AHCT) following thiotepa-based high-dose therapy (HDT) in patients with non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2014; 20: S117.
Tong WP, Ludlum DB . Crosslinking of DNA by busulfan. Formation of diguanyl derivatives. Biochim Biophys Acta 1980; 608: 174–181.
Valdez BC, Murray D, Nieto Y, Li Y, Wang G, Champlin RE et al. Synergistic cytotoxicity of the DNA alkylating agent busulfan, nucleoside analogs and suberoylanilide hydroxamic acid in lymphoma cell lines. Leuk Lymphoma 2012; 53: 973–981.
Schuler US, Renner UD, Kroschinsky F, Johne C, Jenke A, Naumann R et al. Intravenous busulphan for conditioning before autologous or allogeneic human blood stem cell transplantation. Br J Haematol 2001; 114: 944–950.
Madden T, de Lima M, Thapar N, Nguyen J, Roberson S, Couriel D et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant 2007; 13: 56–64.
Dean RM, Pohlman B, Sweetenham JW, Sobecks RM, Kalaycio ME, Smith SD et al. Superior survival after replacing oral with intravenous busulfan in autologous stem cell transplantation for non-Hodgkin lymphoma with busulfan, cyclophosphamide and etoposide. Br J Haematol 2010; 148: 226–234.
Aggarwal C, Gupta S, Vaughan WP, Saylors GB, Salzman DE, Katz RO et al. Improved outcomes in intermediate- and high-risk aggressive non-Hodgkin lymphoma after autologous hematopoietic stem cell transplantation substituting intravenous for oral busulfan in a busulfan, cyclophosphamide, and etoposide preparative regimen. Biol Blood Marrow Transplant 2006; 12: 770–777.
Srivastava A, Bradstock KF, Szer J, de Bortoli L, Gottlieb DJ . Busulphan and melphalan prior to autologous bone marrow transplantation. Bone Marrow Transplant 1993; 12: 323–329.
Lu C, Braine HG, Kaizer H, Saral R, Tutschka PJ, Santos GW . Preliminary results of high-dose busulfan and cyclophosphamide with syngeneic or autologous bone marrow rescue. Cancer Treat Rep 1984; 68: 711–717.
Phillips GL, Shepherd JD, Barnett MJ, Lansdorp PM, Klingemann HG, Spinelli JJ et al. Busulfan, cyclophosphamide, and melphalan conditioning for autologous bone marrow transplantation in hematologic malignancy. J Clin Oncol 1991; 9: 1880–1888.
Copelan EA, Penza SL, Pohlman B, Avalos BR, Goormastic M, Andresen SW et al. Autotransplantation following busulfan, etoposide and cyclophosphamide in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant 2000; 25: 1243–1248.
Hwang DY, Kim SJ, Cheong JW, Kim Y, Jang JE, Lee JY et al. High pre-transplant serum ferritin and busulfan-thiotepa conditioning regimen as risk factors for hepatic sinusoidal obstructive syndrome after autologous stem cell transplantation in patients with malignant lymphoma. Leuk Lymphoma 2015; 57: 1–20.
Shin HJ, Lee WS, Lee HS, Kim H, Lee GW, Song MK et al. Busulfan-containing conditioning regimens are optimal preparative regimens for autologous stem cell transplant in patients with diffuse large B-cell lymphoma. Leuk Lymphoma 2014; 55: 2490–2496.
Nieto Y, Thall P, Valdez B, Andersson B, Popat U, Anderlini P et al. High-dose infusional gemcitabine combined with busulfan and melphalan with autologous stem-cell transplantation in patients with refractory lymphoid malignancies. Biol Blood Marrow Transplant 2012; 18: 1677–1686.
Kang BW, Kim WS, Kim C, Jang G, Lee SS, Choi YH et al. Yttrium-90-ibritumomab tiuxetan in combination with intravenous busulfan, cyclophosphamide, and etoposide followed by autologous stem cell transplantation in patients with relapsed or refractory B-cell non-Hodgkin's lymphoma. Invest New Drugs 2010; 28: 516–522.
Stewart DA, Duan Q, Carlson L, Russell JA, Bahlis NJ, Duggan P et al. A prospective phase II study of RICE re-induction, then high-dose fludarabine and busulfan, followed by autologous or allogeneic blood stem cell transplantation for indolent b-cell lymphoma. Clin Lymphoma Myeloma Leuk 2011; 11: 475–482.
Ahn JS, Yang DH, Jung SH, Chae YS, Sohn SK, Yhim HY et al. Autologous stem cell transplantation with busulfan, cyclophosphamide, and etoposide as an intensifying frontline treatment in patients with peripheral T cell lymphomas: a multicenter retrospective trial. Ann Hematol 2013; 92: 789–797.
Brice P, Divine M, Simon D, Coiffier B, Leblond V, Simon M et al. Feasibility of tandem autologous stem-cell transplantation (ASCT) in induction failure or very unfavorable (UF) relapse from Hodgkin's disease (HD). SFGM/GELA Study Group. Ann Oncol 1999; 10: 1485–1488.
Kim JE, Lee DH, Yoo C, Kim S, Kim SW, Lee JS et al. BEAM or BuCyE high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin's lymphoma patients: a single center comparative analysis of efficacy and toxicity. Leuk Res 2011; 35: 183–187.
Gonzalez-Vicent M, Molina B, Perez A, Diaz MA . Once-daily intravenous busulfan for 47 pediatric patients undergoing autologous hematopoietic stem cell transplantation: a single center study. J Pediatr Hematol Oncol 2012; 34: 180–183.
Blanes M, Lahuerta JJ, Gonzalez JD, Ribas P, Solano C, Alegre A et al. Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant 2013; 19: 69–74.
Blanes M, de la Rubia J . Role of autologous bone marrow transplant in multiple myeloma. Curr Opin Oncol 2012; 24: 733–741.
Nieto Y, Popat U, Anderlini P, Valdez B, Andersson B, Liu P et al. Autologous stem cell transplantation for refractory or poor-risk relapsed Hodgkin's lymphoma: effect of the specific high-dose chemotherapy regimen on outcome. Biol Blood Marrow Transplant 2013; 19: 410–417.
Caballero MD, Rubio V, Rifon J, Heras I, Garcia-Sanz R, Vazquez L et al. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant 1997; 20: 451–458.
Sakellari I, Mallouri D, Batsis I, Apostolou C, Konstantinou V, Abela EM et al. Carmustine, etoposide, cytarabine and melphalan versus a newly designed intravenous busulfan-based Busulfex, etoposide and melphalan conditioning regimen for autologous hematopoietic cell transplant: a retrospective matched-pair analysis in advanced Hodgkin and non-Hodgkin lymphomas. Leuk Lymphoma 2015; 56: 3071–3081.
Cheng T, Forsyth P, Chaudhry A, Morris D, Gluck S, Russell JA et al. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transplant 2003; 31: 679–685.
Alimohamed N, Daly A, Owen C, Duggan P, Stewart DA . Upfront thiotepa, busulfan, cyclophosphamide, and autologous stem cell transplantation for primary CNS lymphoma: a single centre experience. Leuk Lymphoma 2012; 53: 862–867.
Soussain C, Choquet S, Fourme E, Delgadillo D, Bouabdallah K, Ghesquieres H et al. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica 2012; 97: 1751–1756.
Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol 2008; 26: 2512–2518.
Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015; 125: 1403–1410.
Welch MR, Sauter CS, Matasar MJ, Faivre G, Weaver SA, Moskowitz CH et al. Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide. Leuk Lymphoma 2015; 56: 361–367.
Cote GM, Hochberg EP, Muzikansky A, Hochberg FH, Drappatz J, McAfee SL et al. Autologous stem cell transplantation with thiotepa, busulfan, and cyclophosphamide (TBC) conditioning in patients with CNS involvement by non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2012; 18: 76–83.
Ganguly S, Jain V, Divine C, Aljitawi O, Abhyankar S, McGuirk J . BU, melphalan and thiotepa as a preparative regimen for auto-transplantation in Hodgkin's disease. Bone Marrow Transplant 2012; 47: 311–312.
Montemurro M, Kiefer T, Schuler F, Al-Ali HK, Wolf HH, Herbst R et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol 2007; 18: 665–671.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
GD received an unrestricted research grant from Mundipharma. The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Damaj, G., Cornillon, J., Bouabdallah, K. et al. Carmustine replacement in intensive chemotherapy preceding reinjection of autologous HSCs in Hodgkin and non-Hodgkin lymphoma: a review. Bone Marrow Transplant 52, 941–949 (2017). https://doi.org/10.1038/bmt.2016.340
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.340
This article is cited by
-
XRCC1 399GG genotype predicts significantly longer overall survival in resistant lymphoma patients treated with Benda-EAM and ASCT
Bone Marrow Transplantation (2020)
-
CBeV (cyclophosphamide, bendamustine and etoposide) pre-autotransplant conditioning in persons with lymphoma
Bone Marrow Transplantation (2020)
-
Bendamustine-EAM versus BEAM regimen in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in the frontline setting: a multicenter retrospective study from Lymphoma Study Association (LYSA) centers
Bone Marrow Transplantation (2020)
-
LEAM versus CBV for conditioning in autologous hematopoietic stem cell transplantation for lymphoma
Bone Marrow Transplantation (2019)
-
BAM conditioning before autologous transplantation for lymphoma: a study on behalf of the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC)
Annals of Hematology (2019)