Abstract
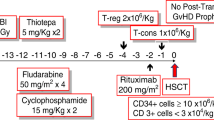
Between 2012 and 2015, 42 pediatric patients underwent haploidentical hematopoietic cell transplantation using an αβ+ T-cell-depleted graft with targeted αβ cells at 1–5 × 105/kg by add-back; 31 had hematologic malignancy (HM), 8 had non-malignant disease (NM) and 3 had solid tumors. All patients received uniform reduced-intensity conditioning with fludarabine, cyclophosphamide, rabbit anti-thymocyte globulin and low-dose TBI. All 42 patients achieved neutrophil engraftment at a median of 10 days. The cumulative incidences (CIs) of ⩾grade II and ⩾grade III acute GvHD were 31±7.1% (SE) and 12±5.0%, respectively, and 1-year CI of chronic GvHD was 15±5.8%. One patient died of CMV pneumonia, leading to transplant-related mortality (TRM) of 2.6±2.5%. Sixteen patients relapsed and 11 died of disease. At a median follow-up of 19 months (range, 5–43 months), the estimated 2-year event-free survival for NM and HM were 88±11.7 and 50±10.1%, respectively. Our study demonstrated that haploidentical hematopoietic cell transplantation after ex vivo depletion of αβ+ T cells with targeted dose noticeably reduced the graft failure rate and TRM in pediatric patients and could be applied to patients lacking a suitable related or unrelated donor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lang P, Schumm M, Greil J, Bader P, Klingebiel T, Muller I et al. A comparison between three graft manipulation methods for haploidentical stem cell transplantation in pediatric patients: preliminary results of a pilot study. Klin Padiatr 2005; 217: 334–338.
Bethge WA, Faul C, Bornhauser M, Stuhler G, Beelen DW, Lang P et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis 2008; 40: 13–19.
Handgretinger R, Chen X, Pfeiffer M, Mueller I, Feuchtinger T, Hale GA et al. Feasibility and outcome of reduced-intensity conditioning in haploidentical transplantation. Ann NY Acad Sci 2007; 1106: 279–289.
Airoldi I, Bertaina A, Prigione I, Zorzoli A, Pagliara D, Cocco C et al. gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood 2015; 125: 2349–2358.
Lang P, Feuchtinger T, Teltschik HM, Schwinger W, Schlegel P, Pfeiffer M et al. Improved immune recovery after transplantation of TCRalphabeta/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transplant 2015; 50 (Suppl 2): S6–S10.
Daniele N, Scerpa MC, Caniglia M, Bernardo ME, Rossi C, Ciammetti C et al. Transplantation in the onco-hematology field: focus on the manipulation of alphabeta and gammadelta T cells. Pathol Res Pract 2012; 208: 67–73.
Im HJ, Koh KN, Suh JK, Lee SW, Choi ES, Jang S et al. Refinement of treatment strategies in ex vivo T-cell-depleted haploidentical SCT for pediatric patients. Bone Marrow Transplant 2015; 50: 225–231.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Lamb LS Jr, Lopez RD . Gammadelta T cells: a new frontier for immunotherapy? Biol Blood Marrow Transplant 2005; 11: 161–168.
Locatelli F, Bauquet A, Palumbo G, Moretta F, Bertaina A . Negative depletion of alpha/beta+ T cells and of CD19+ B lymphocytes: a novel frontier to optimize the effect of innate immunity in HLA-mismatched hematopoietic stem cell transplantation. Immunol Lett 2013; 155: 21–23.
Norell H, Moretta A, Silva-Santos B, Moretta L . At the bench: preclinical rationale for exploiting NK cells and gammadelta T lymphocytes for the treatment of high-risk leukemias. J Leukoc Biol 2013; 94: 1123–1139.
Minculescu L, Sengelov H . The role of gamma delta T cells in haematopoietic stem cell transplantation. Scand J Immunol 2015; 81: 459–468.
Handgretinger R . New approaches to graft engineering for haploidentical bone marrow transplantation. Semin Oncol 2012; 39: 664–673.
Schumm M, Lang P, Bethge W, Faul C, Feuchtinger T, Pfeiffer M et al. Depletion of T-cell receptor alpha/beta and CD19 positive cells from apheresis products with the CliniMACS device. Cytotherapy 2013; 15: 1253–1258.
Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood 2014; 124: 822–826.
Lang P, Teltschik HM, Feuchtinger T, Muller I, Pfeiffer M, Schumm M et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol 2014; 165: 688–698.
Maschan M, Shelikhova L, Ilushina M, Kurnikova E, Boyakova E, Balashov D et al. TCR-alpha/beta and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone Marrow Transplant 2016; 51: 668–674.
Wolff SN . Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplant 2002; 29: 545–552.
Storb R, Prentice RL, Thomas ED, Appelbaum FR, Deeg HJ, Doney K et al. Factors associated with graft rejection after HLA-identical marrow transplantation for aplastic anaemia. Br J Haematol 1983; 55: 573–585.
Bacigalupo A . Hematopoietic stem cell transplants after reduced intensity conditioning regimen (RI-HSCT): report of a workshop of the European group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 2000; 25: 803–805.
Baron F, Baker JE, Storb R, Gooley TA, Sandmaier BM, Maris MB et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 2004; 104: 2254–2262.
McDonough CH, Jacobsohn DA, Vogelsang GB, Noga SJ, Chen AR . High incidence of graft failure in children receiving CD34+ augmented elutriated allografts for nonmalignant diseases. Bone Marrow Transplant 2003; 31: 1073–1080.
Ljungman P, Brand R, Hoek J, de la Camara R, Cordonnier C, Einsele H et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis 2014; 59: 473–481.
Servais S, Dumontier N, Biard L, Schnepf N, Resche-Rigon M, de Latour RP et al. Response to antiviral therapy in hematopoietic stem cell transplant recipients with cytomegalovirus (CMV) reactivation according to the donor CMV serologic status. Clin Microbiol Infect 2016; 22: 289.e1–7.
Schlegel P, Feuchtinger T, Nitschke-Gerard C, Seidel UJ, Lang AM, Kyzirakos C et al. Favorable NK cell activity after haploidentical hematopoietic stem cell transplantation in stage IV relapsed Ewing's sarcoma patients. Bone Marrow Transplant 2015; 50 (Suppl 2): S72–S76.
Acknowledgements
This study was supported by a grant (2012–0829) from the Asan Institute for Life Sciences, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Im, H., Koh, K., Suh, J. et al. Haploidentical HCT using an αβ T-cell-depleted graft with targeted αβ+ cells by add-back after a reduced intensity preparative regimen containing low-dose TBI. Bone Marrow Transplant 51, 1217–1222 (2016). https://doi.org/10.1038/bmt.2016.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.114
This article is cited by
-
The role of interim-foscarnet prophylaxis in preventing cytomegalovirus infection after ex vivo αβ T cell-depleted haploidentical hematopoietic cell transplant in children
Bone Marrow Transplantation (2021)
-
Improved outcomes of allogeneic hematopoietic stem cell transplantation including haploidentical transplantation for childhood myelodysplastic syndrome
Bone Marrow Transplantation (2020)