Abstract
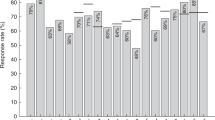
The guidelines for immunization of hematopoietic SCT (HSCT) recipients recommend three doses of antipneumococcal conjugate vaccine (PCV) from 3 to 6 months after transplant, followed by a dose of polysaccharide 23-valent (PPV23) vaccine at 12 months in the case of no GVHD or an additional PCV dose in the case of GVHD. Due to the lack of long-term data in the literature, there is no recommendation for boosts after 12 months. Our goal was to assess the maintenance of the immune response to pneumococcal vaccines in patients vaccinated 10 years ago according to current guidelines. Thirty surviving patients of the IDWP01 (Infectious Diseases Working Party 1) trial were assessed for antibody levels against the seven antigens of the PCV7 and against two of the PPV23-specific antigens. When compared with 24 months after transplant, the immune response did not significantly decrease but with important serotype-specific variability. There was no evidence that an additional dose of PPV23 given to 11/30 patients 2–11 years after transplant was beneficial. In long-term HSCT survivors with no or few GVHD vaccinated against Streptococcus pneumoniae according to the current guidelines, the specific immunity is not fully maintained a decade later. The optimal schedule of antipneumococcal vaccination in HSCT recipients after 12 months remains to be established.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Engelhard D, Cordonnier C, Shaw PJ, Parkalli T, Guenther C, Martino R et al. Early and late invasive pneumococcal infection following stem cell transplantation: a European Bone Marrow Transplantation survey. Br J Haematol 2002; 117: 444–450.
Giebink GS, Warkentin PI, Ramsay NK, Kersey JH . Titers of antibody to pneumococci in allogeneic bone marrow transplant recipients before and after vaccination with pneumococcal vaccine. J Infect Dis 1986; 154: 590–596.
Youssef S, Rodriguez G, Rolston KV, Champlin RE, Raad II, Safdar A . Streptococcus pneumoniae infections in 47 hematopoietic stem cell transplantation recipients: clinical characteristics of infections and vaccine-breakthrough infections, 1989-2005. Medicine (Baltimore) 2007; 86: 69–77.
Ljungman P, Cordonnier C, Einsele H, Englund J, Machado CM, Storek J et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant 2009; 44: 521–526.
Rubin L, Levin M, Ljungman P, Davies E, Avery R, Tomblun M et al. 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin Infect Dis 2014; 58: 309–318.
Cordonnier C, Labopin M, Chesnel V, Ribaud P, de la Camara R, Martino R et al. Immune response to the 23-valent polysaccharide vaccine after the 7-valent conjugate vaccine in allogeneic stem cell transplant recipients: Results from the EBMT IDWP01 trial. Vaccine 2010; 28: 2730–2734.
Cordonnier C, Labopin M, Chesnel V, Ribaud P, De La Camara R, Martino R et al. Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis 2009; 48: 1392–1401.
Cordonnier C, Labopin M, Jansen KU, Pride M, Chesnel V, Bonnet E et al. Relationship between IgG titers and opsonocytophagocytic activity of anti-pneumococcal antibodies after immunization with the 7-valent conjugate vaccine in allogeneic stem cell transplant. Bone Marrow Transplant 2010; 45: 1423–1426.
Meisel R, Kuypers L, Dirksen U, Schubert R, Gruhn B, Strauss G et al. Pneumococcal conjugate vaccine provides early protective antibody responses in children after related and unrelated allogeneic hematopoietic stem cell transplantation. Blood 2007; 109: 2322–2326.
Molrine DC, Antin JH, Guinan EC, Soiffer RJ, MacDonald K, Malley R et al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood 2003; 101: 831–836.
Esposito S, Tansey S, Thompson A, Razmpour A, Liang J, Jones TR et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin Vaccine Immunol 2010; 17: 1017–1026.
Concepcion NF, Frasch CE . Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2001; 8: 266–272.
Musher DM, Manoff SB, McFetridge RD, Liss CL, Marchese RD, Raab J et al. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Hum Vaccin 2011; 7: 919–928.
Kumar D, Welsh B, Siegal D, Chen MH, Humar A . Immunogenicity of pneumococcal vaccine in renal transplant recipients—three year follow-up of a randomized trial. Am J Transplant 2007; 7: 633–638.
Barra A, Cordonnier C, Preziosi MP, Intrator L, Hessel L, Fritzell B et al. Immunogenicity of Haemophilus influenzae type b conjugate vaccine in allogeneic bone marrow recipients. J Infect Dis 1992; 166: 1021–1028.
Kumar D, Chen MH, Welsh B, Siegal D, Cobos I, Messner HA et al. A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients. Clin Infect Dis 2007; 45: 1576–1582.
Blum MD, Dagan R, Mendelman PM, Pinsk V, Giordani M, Li S et al. A comparison of multiple regimens of pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine and pneumococcal polysaccharide vaccine in toddlers. Vaccine 2000; 18: 2359–2367.
de Roux A, Schmole-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008; 46: 1015–1023.
O'Brien KL, Hochman M, Goldblatt D . Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis 2007; 7: 597–606.
Acknowledgements
We are grateful to Luca Mollo, MD (Medical Director Vaccines, Pfizer, France) and Veronique Schindler (IIR Manager, Pfizer France), Linda Xu (Data management and logistics, Vaccines R and D, Pfizer Laboratories, Pearl River, US) and Adriana Cahill (WW Research &Development, Vaccines R and D, Pfizer Laboratories, Pearl River, US) for their help in the organization of the study and the laboratory assessments. We also thank the staff of the Plateforme de Ressources Biologiques of Henri Mondor University Hospital, especially Dr Bijan Ghaleh and Dr Caroline Barau, and Dr Oumedaly Reman, Caen University Hospital. This work was supported by Pfizer France.
Author contributions
CC and PL: study concept and design, funding, acquisition of data, analysis and interpretation of data and drafting the manuscript. ML: statistical analysis and drafting the manuscript. PR: study concept, acquisition of data and analysis and interpretation of data. CR, LC and CCh: data collection and analysis and interpretation of data. SC: critical revision of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Dr Cordonnier served on Pfizer advisory boards and speaker bureau and received research funding for her department. Dr Ribaud served on Pfizer advisory boards and speaker bureau. Dr Ljungman has been an investigator on a subsequent Pfizer sponsored study and participated on behalf of his department in a Pfizer advisory board. The remaining authors declare no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Cordonnier, C., Labopin, M., Robin, C. et al. Long-term persistence of the immune response to antipneumococcal vaccines after Allo-SCT: 10-year follow-up of the EBMT-IDWP01 trial. Bone Marrow Transplant 50, 978–983 (2015). https://doi.org/10.1038/bmt.2015.42
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.42
This article is cited by
-
A Review of Vaccinations in Adult Patients with Secondary Immunodeficiency
Infectious Diseases and Therapy (2021)
-
Humoral immune response to tick-borne encephalitis vaccination in allogeneic blood and marrow graft recipients
npj Vaccines (2020)
-
Measuring the cellular memory B cell response after vaccination in patients after allogeneic stem cell transplantation
Annals of Hematology (2020)
-
Serologic response to pneumococcal vaccination in children experiencing recurrent invasive pneumococcal disease
BMC Infectious Diseases (2018)
-
Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: new considerations for a persistent nemesis
Bone Marrow Transplantation (2017)