Abstract
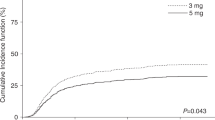
There is significant variability in the serum concentrations of tacrolimus attained early post transplant due to drug interactions and genomic variation. We evaluated whether tacrolimus concentrations early post transplant correlated with incidence of acute GvHD in 120 consecutive patients allografted with a uniform reduced-intensity conditioning regimen. All patients received standard prophylaxis with oral tacrolimus and IV methotrexate. The primary variable of interest was mean weekly tacrolimus concentrations in the initial 4 weeks post transplant. In multivariate analysis, week 1 tacrolimus concentration was an independent predictor of acute grade 2–4 GvHD (hazard ratio (HR), 0.90; 95% confidence interval (CI), 0.84–0.97; P<0.01). This association was driven by a lower risk of acute grade 2–4 GvHD in patients with week 1 tacrolimus concentrations >12 ng/mL (HR, 0.47; 95% CI, 0.25–0.88; P=0.02). Week 1 tacrolimus concentrations were not associated with chronic GvHD, relapse or overall survival. Lower tacrolimus concentrations at weeks 2, 3 and 4 were not associated with a higher incidence of GvHD. In summary, we found that higher tacrolimus concentrations during the first week after allografting with a reduced-intensity conditioning regimen were associated with significantly reduced risk of acute grade 2–4 GvHD without increasing risk of relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Reshef R, Porter DL . Reduced-intensity conditioned allogeneic SCT in adults with AML. Bone Marrow Transplant 2015; 50: 759–769.
Jagasia M, Arora M, Flowers EM, Chao NJ, McCarthy PL, Cutler CS et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012; 119: 296–307.
Eapen M, Logan BR, Horowitz MM, Zhong X, Perales MA, Lee SJ et al. Bone marrow or peripheral blood for reduced-intensity conditioning unrelated donor transplantation. J Clin Oncol 2015; 33: 364–369.
Johnston L . Acute graft-versus-host disease: differing risk with differing graft sources and conditioning intensity. Best Pract Res Clin Haematol 2008; 21: 177–192.
Choi SW, Reddy P . Current and emerging strategies for the prevention of graft-versus host disease. Nat Rev Clin Oncol 2014; 11: 536–547.
Jacobson P, Ng J, Ratanatharathorn V, Uberti J, Brundage RC . Factors affecting the pharmacokinetics of tacrolimus (FK506) in hematopoietic cell transplant (HCT) patients. Bone Marrow Transplant 2001; 28: 753–758.
Onizuka M, Kunii N, Toyosaki M, Machida S, Ohgiya D, Ogawa Y et al. Cytochrome P450 genetic polymorphisms influence the serum concentration of calcineurin inhibitors in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant 2011; 46: 1113–1117.
Brown NW, Gonde CE, Adams JE, Tredger JM . Low hematocrit and serum albumin concentrations underlie the overestimation of tacrolimus concentrations by microparticle enzyme immunoassay versus liquid chromatography-tandem mass spectrometry. Clin Chem 2005; 51: 586–592.
Miano TA, Ganetsky A, Porter DL, Reshef R . Serum hemoglobin is a predictor of tacrolimus whole blood concentration in hematopoietic stem cell transplant patients. 2015 American Society for Clinical Pharmacology and Therapeutics Annual Meeting. 1 February 2015; Wiley-Blackwell. 2015, pp S80–S80.
Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 2005; 106: 1113–1122.
Ram R, Storer B, Mielcarek M, Sandmaier BM, Maloney DG, Martin PJ et al. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 414–422.
Offer K, Kolb M, Jin Z, Bhatia M, Kung AL, George D et al. Efficacy of tacrolimus/mycophenolate mofetil as acute graft-versus-host disease prophylaxis and the impact of subtherapeutic tacrolimus levels in children after matched sibling donor allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2015; 21: 496–502.
Mori T, Kato J, Shimizu T, Aisa Y, Nakazato T, Yamane A et al. Effect of early posttransplantation tacrolimus concentration on the development of acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation from unrelated donors. Biol Blood Marrow Transplant 2012; 18: 229–234.
Rogosheske JR, Fargen AD, DeFor TE, Warlick E, Arora M, Blazar BR et al. Higher therapeutic CsA levels early post-transplantation reduces risks of acute graft-versus-host disease and improves survival. Bone Marrow Transplant 2014; 49: 122–125.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U et al. International, multi-center standardization of acute graft-versus-host disease clinical data collection: a report from the MAGIC consortium. Biol Blood Marrow Transplant (e-pub ahead of print 16 September 2015; doi:10.1016/j.bbmt.2015.09.001).
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood 2014; 123: 3664–3671.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P . Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204–R212.
Przepiorka D, Nash RA, Wingard JR, Zhu J, Maher RM, Fitzsimmons WE et al. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation. Biol Blood Marrow Transplant 1999; 5: 94–97.
Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol 2003; 4: 154–160.
Sun K, Wilkins DEC, Anver MR, Sayers TJ, Panoskaltsis-Mortari A, Blazar BR et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood 2005; 106: 3293–3299.
Luznik L, O’Donnell PV, Fuchs EJ . Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical BMT. Semin Oncol 2012; 39: 1–16.
Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T . The role of pharmacogenetics in the disposition of and response of tacrolimus in solid organ transplantation. Clin Pharmacokinet 2014; 53: 123–139.
Acknowledgements
This work was supported by a Career Development Award from the Conquer Cancer Foundation (RR); Amy Strelzer Manasevit Award from the National Marrow Donor Program (RR); National Institutes of Health grants K23-CA178202 (RR) & U01-HL069286 (DLP), and the Margie and Andy Rooke Fund for Leukemia Research (RR and DLP). We thank Oren Litvin for help with preparation of the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ganetsky, A., Shah, A., Miano, T. et al. Higher tacrolimus concentrations early after transplant reduce the risk of acute GvHD in reduced-intensity allogeneic stem cell transplantation. Bone Marrow Transplant 51, 568–572 (2016). https://doi.org/10.1038/bmt.2015.323
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.323
This article is cited by
-
Tacrolimus initial steady state level in post-transplant cyclophosphamide-based GvHD prophylaxis regimens
Bone Marrow Transplantation (2022)
-
Intrapatient variability in concentration/dose ratio of tacrolimus predicts transplant-associated thrombotic microangiopathy
International Journal of Hematology (2021)
-
Lack of a significant pharmacokinetic interaction between letermovir and calcineurin inhibitors in allogeneic HCT recipients
Bone Marrow Transplantation (2020)
-
Low mean post-transplantation tacrolimus levels in weeks 2–3 correlate with acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation from related and unrelated donors
Bone Marrow Transplantation (2019)
-
Allo-HSCT recipients with invasive fungal disease and ongoing immunosuppression have a high risk for developing tuberculosis
Scientific Reports (2019)