Abstract
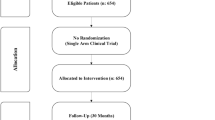
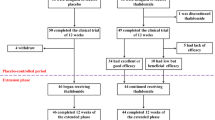
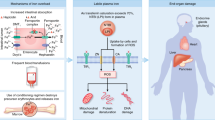
Elevated serum ferritin contributes to treatment-related morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT). The multicenter DE02 trial assessed the safety, efficacy and impact of deferasirox on iron homeostasis after allogeneic HSCT. Deferasirox was administered at a starting dose of 10 mg/kg per day to 76 recipients of allogeneic HSCT, with subsequent dose adjustments based on efficacy and safety. Deferasirox was initiated at a median of 168 days after HSCT, with 84% of patients still on immunosuppression. Baseline serum ferritin declined from 2045 to 957 ng/mL. Deferasirox induced a negative iron balance in 84% of patients. Hemoglobin increased in the first 3 months, and trough serum cyclosporine levels were stable. Median exposure was 330 days, with a median compliance rate of >80%. The most common investigator-reported drug-related adverse events (AEs) were increased blood creatinine (26.5%), nausea (9.0%) and abdominal discomfort (8.3%). Fifty-four (71.1%) patients experienced drug-related AEs, which occasionally resulted in discontinuation (gastrointestinal (n=6), skin (n=3), elevated transaminases (n=1) and creatinine (n=1)). The incidence of AEs appeared to be dose related, with 7.5 mg/kg per day being the best-tolerated dose. Low-dose deferasirox is an effective chelation therapy after allogeneic HSCT, with a manageable safety profile, even in patients receiving cyclosporine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Malcovati L . Impact of transfusion dependency and secondary iron overload on the survival of patients with myelodysplastic syndromes. Leuk Res 2007; 31: S2–S6.
Majhail NS, Lazarus HM, Burns LJ . Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant 2008; 41: 997–1003.
Butt NM, Clark RE . Autografting as a risk factor for persisting iron overload in long-term survivors of acute myeloid leukaemia. Bone Marrow Transplant 2003; 32: 909–913.
Lichtman SM, Attivissimo L, Goldman IS, Schuster MW, Buchbinder A . Secondary hemochromatosis as a long-term complication of the treatment of hematologic malignancies. Am J Hematol 1999; 61: 262–264.
Chotsampancharoen T, Gan K, Kasow KA, Barfield RC, Hale GA, Leung W . Iron overloading survivors of childhood leukemia after allogeneic hematopoietic stem cell transplantation. Pediatr Transplant 2008; 13: 348–352.
Ganz T, Nemeth E . Hepcidin and disorders of iron metabolism. Annu Rev Med 2011; 62: 347–360.
Meyer SC, O'Meara A, Buser AS, Tichelli A, Passweg JR, Stern M . Prognostic impact of posttransplantation iron overload after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2013; 19: 440–444.
Großekatthöfer M, Güclü ED, Lawitschka A, Matthes-Martin S, Mann G, Minkov M et al. Ferritin concentrations correlate to outcome of hematopoietic stem cell transplantation but do not serve as biomarker of graft-versus-host disease. Ann Hematol 2013; 92: 1121–1128.
Tomás JF, Pinilla I, García-Buey ML, García A, Figuera A, Gómez-García de Soria VGG et al. Long-term liver dysfunction after allogeneic bone marrow transplantation: clinical features and course in 61 patients. Bone Marrow Transplant 2000; 26: 649–655.
McDonald GB . Review article: management of hepatic disease following haematopoietic cell transplant. Aliment Pharmacol Ther 2006; 24: 441–452.
Sucak GT, Yegin ZA, Özkurt ZN, Aki SZ, Karakan T, Akyol G . The role of liver biopsy in the workup of liver dysfunction late after SCT: is the role of iron overload underestimated? Bone Marrow Transplant 2008; 42: 461–467.
Eisfeld AK, Westerman M, Krahl R, Leiblein S, Liebert UG, Hehme M et al. Highly elevated serum hepcidin in patients with acute myeloid leukemia prior to and after allogeneic hematopoietic cell transplantation: does this protect from excessive parenchymal iron loading? Adv Hematol 2011; 2011: 491058.
Lee JW, Kang HJ, Kim EK, Kim H, Shin HY, Ahn HS . Effect of iron overload and iron-chelating therapy on allogeneic hematopoietic SCT in children. Bone Marrow Transplant 2009; 44: 793–797.
Meo A, Ruggeri A, La Rosa MA, Zanghi L, Morabito N, Duca L . Iron burden and liver fibrosis decrease during a long-term phlebotomy program and iron chelating treatment after bone marrow transplantation. Hemoglobin 2006; 30: 131–137.
Majhail NS, Lazarus HM, Burns LJ . A prospective study of iron overload management in allogeneic hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant 2010; 16: 832–837.
Eisfeld AK, Krahl R, Jaekel N, Niederwieser D, Al-Ali HK . Kinetics of iron removal by phlebotomy in patients with iron overload after allogeneic hematopoietic cell transplantation. Am J Blood Res 2012; 2: 243–253.
Inati A, Sbeiti N, Khoriaty E, Cappellini MD, Koussa S, Nasr TA et al. 1-year results from a prospective randomized trial comparing phlebotomy with deferasirox for the treatment of iron overload in pediatric patients with thalassemia major following curative stem cell transplantation. Blood 2011; 118; abstract 904.
Li CK, Lai DH, Shing MM, Chik KW, Lee V, Yuen PM . Early iron reduction programme for thalassaemia patients after bone marrow transplantation. Bone Marrow Transplant 2000; 25: 653–656.
Sivgin S, Eser B, Bahcebasi S, Kaynar L, Kurnaz F, Uzer E et al. Efficacy and safety of oral deferasirox treatment in the posttransplant period for patients who have undergone allogeneic hematopoietic stem cell transplantation (alloHSCT). Ann Hematol 2011; 91: 743–749.
Sivgin S, Baldane S, Akyol G, Keklik M, Kaynar L, Kurnaz F et al. The oral iron chelator deferasirox might improve survival in allogeneic hematopoietic cell transplant (alloHSCT) recipients with transfusional iron overload. Transfus Apher Sci 2013; 49: 295–301.
Gaziev D, Giardini C, Angelucci E, Polchi P, Galimberti M, Baronciani D et al. Intravenous chelation therapy during transplantation for thalassemia. Haematologica 1995; 80: 300–304.
Cappellini MD, Porter JB, El-Beshlawy A, Li C-K, Seymour JF, Elalfy M et al. Tailoring iron chelation by iron intake and serum ferritin trends: the prospective multicenter EPIC study of deferasirox in 1744 patients with various transfusion-dependent anemias. Haematologica 2010; 95: 557–566.
Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1- yr prospective study. Eur J Haematol 2008; 80: 168–176.
Gattermann N, Finelli C, Della Porta M, Fenaux P, Ganser A, Guerci-Bresler A et al. Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: Results from the large 1-year EPIC study. Leuk Res 2010; 34: 1143–1150.
Lee J-W, Yoon S-S, Shen ZX, Ganser A, Hsu H-C, Habr D et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood 2010; 116: 2448–2454.
Cappellini MD, Bejaoui M, Agaoglu L, Canatan D, Capra M, Cohen A et al. Iron chelation with deferasirox in adult and pediatric patients with thalassemia major: efficacy and safety during 5 years' follow-up. Blood 2011; 118: 884–893.
Taher AT, Porter J, Viprakasit V, Kattamis A, Chuncharunee S, Sutcharitchan P et al. Deferasirox significantly reduces iron overload in non-transfusion-dependent thalassemia: 1-year results from a prospective, randomized, double-blind, placebo-controlled study. Blood 2012; 120: 970–977.
Taher AT, Porter JB, Viprakasit V, Kattamis A, Chuncharunee S, Sutcharitchan P et al. Deferasirox effectively reduces iron overload in non-transfusion-dependent thalassemia (NTDT) patients: 1-year extension results from the THALASSA study. Ann Hematol 2013; 92: 1485–1493.
Altamura S, Kiss J, Blattmann C, Gilles W, Muckenthaler MU . SELDI-TOF MS detection of urinary hepcidin. Biochimie 2009; 91: 1335–1338.
Vallejo C, Batlle M, Vazquez L, Solano C, Sampol A, Duarte R et al. Phase IV open-label study of the efficacy and safety of deferasirox after allogeneic stem cell transplantation. Haematologica 2014; 99: 1632–1637.
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004; 306: 2090–2093.
Kroot JJ, van Herwaarden AE, Tjalsma H, Jansen RT, Hendriks JC, Swinkels DW . Second round robin for plasma hepcidin methods: first steps toward harmonization. Am J Hematol 2012; 87: 977–983.
Pantopoulos K . Function of the hemochromatosis protein HFE: lessons from animal models. World J Gastroenterol 2008; 14: 6893–6901.
van Dijk BA, Laarakkers CM, Klaver SM, Jacobs EM, van Tits LJ, Janssen MC et al. Serum hepcidin levels are innately low in HFE-related haemochromatosis but differ between C282Y-homozygotes with elevated and normal ferritin levels. Br J Haematol 2008; 142: 979–985.
Piperno A, Girelli D, Nemeth E, Trombini P, Bozzini C, Poggiali E et al. Blunted hepcidin response to oral iron challenge in HFE-related hemochromatosis. Blood 2007; 110: 4096–4100.
Pedersen P, Milman N . Genetic screening for HFE hemochromatosis in 6,020 Danish men: penetrance of C282Y, H63D, and S65C variants. Ann Hematol 2009; 88: 775–784.
Khoury MJ, McCabe LL, McCabe ER . Population screening in the age of genomic medicine. N Engl J Med 2003; 348: 50–58.
Vujic SM, Kiss J, Herrmann T, Galy B, Martinache S, Stolte J et al. Hfe acts in hepatocytes to prevent hemochromatosis. Cell Metab 2008; 7: 173–178.
Gattermann N, Finelli C, Della PM, Fenaux P, Stadler M, Guerci-Bresler A et al. Hematologic responses with deferasirox therapy in transfusion-dependent myelodysplastic syndromes patients. Haematologica 2012; 97: 1364–1371.
Improta S, Villa MR, Volpe A, Lombardi A, Stiuso P, Cantore N et al. Transfusion-dependent low-risk myelodysplastic patients receiving deferasirox: Long-term follow-up. Oncol Lett 2013; 6: 1774–1778.
Daar S, Pathare A, Nick H, Kriemler-Krahn U, Hmissi A, Habr D et al. Reduction in labile plasma iron during treatment with deferasirox, a once-daily oral iron chelator, in heavily iron-overloaded patients with -thalassaemia. Eur J Haematol 2009; 82: 454–457.
Saigo K, Kono M, Takagi Y, Takenokuchi M, Hiramatsu Y, Tada H et al. Deferasirox reduces oxidative stress in patients with transfusion dependency. J Clin Med Res 2013; 5: 57–60.
Ghoti H, Fibach E, Westerman M, Gordana O, Ganz T, Rachmilewitz EA . Increased serum hepcidin levels during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndrome. Br J Haematol 2011; 153: 118–120.
Skerjanec A, Wang J, Maren K, Rojkjaer L . Investigation of the pharmacokinetic interactions of deferasirox, a once-daily oral iron chelator, with midazolam, rifampin, and repaglinide in healthy volunteers. J Clin Pharmacol 2010; 50: 205–213.
Metzgeroth G, Dinter D, Schultheis B, Dorn-Beineke A, Lutz K, Leismann O et al. Deferasirox in MDS patients with transfusion-caused iron overload-a phase-II study. Ann Hematol 2009; 88: 301–310.
List AF, Baer MR, Steensma DP, Raza A, Esposito J, Martinez-Lopez N et al. Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J Clin Oncol 2012; 30: 2134–2139.
Al-Ali HK, Bourgeois M, Krahl R, Edel E, Leiblein S, Poenisch W et al. The impact of the age of HLA-identical siblings on mobilization and collection of PBSCs for allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2011; 46: 1296–1302.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol 1991; 28: 250–259.
Glucksberg H, Storb R, Fever A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA matched sibling donors. Transplantation 1974; 18: 295–304.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. 1980) Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutesof Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015; 21: 389–401.
Armand P, Kim HT, Virtanen JM, Parkkola RK, Itälä-Remes MA, Majhail NS et al. Iron overload in allogeneic hematopoietic cell transplantation outcome: a meta-analysis. Biol Blood Marrow Transplant 2014; 20: 1248–1251.
Acknowledgements
This study was funded by Novartis Pharma AG. We thank Rebecca Helson and Catherine Risebro of Mudskipper Business Ltd for medical editorial assistance. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Author contributions
NJ: Trial conduct and manuscript writing. GB, NK, UP, MS, KdH and DN: Trial conduct. KH: Data collection. SA and MUM: Hepcidin analysis. KL: Data collection and monitoring. SA and OL: Study design, protocol writing. HKA-A: Study design, protocol writing, trial conduct and manuscript writing. All authors participated in data interpretation and critical review of the manuscript, approved the manuscript and the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
NJ, KH, MS, KdH, SA: None declared. GB: Research funding, travel grants and honoraria, Novartis. NK: Research funding, Novartis. UP: Research funding and honoraria, Novartis. MUM: Consulting fees and payment for the hepcidin analyses, Novartis. DN: Research funding and honoraria, Novartis. HKA-A: Research funding and honoraria, Novartis. KL, SA, OL: Employment, Novartis.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Jaekel, N., Lieder, K., Albrecht, S. et al. Efficacy and safety of deferasirox in non-thalassemic patients with elevated ferritin levels after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 51, 89–95 (2016). https://doi.org/10.1038/bmt.2015.204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.204
This article is cited by
-
ABCC2 c.-24 C>T single-nucleotide polymorphism was associated with the pharmacokinetic variability of deferasirox in Chinese subjects
European Journal of Clinical Pharmacology (2020)
-
Efficacy and safety of oral deferasirox treatment for transfusional iron overload in pure red cell aplasia patients after allogeneic stem cell transplantation
Annals of Hematology (2019)
-
Deferasirox for the treatment of iron overload after allogeneic hematopoietic cell transplantation: multicenter phase I study (KSGCT1302)
International Journal of Hematology (2018)