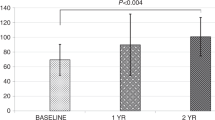
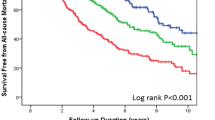
Abstract
Estimating prognosis in sickle cell anemia (SCA) assumes greater importance as intensive treatments, such as hematopoietic SCT (HSCT), are being tested. Here we estimate the mortality risk from the walk-PHaSST (Sildenafil Therapy for Pulmonary Hypertension and Sickle Cell Disease) trial of homozygous SCA patients with suspected pulmonary hypertension (19/468 deaths; 10 centers in the US and UK). Parallel investigations were also undertaken in the Cooperative Study of Sickle Cell Disease (CSCCD) and a contemporary urban sickle cell disease population (Case Western Reserve University-University Hospitals (CWRU-UH), Cleveland, OH, USA). One- and two-value positive predictive values for 2-year mortality (from study entry) are calculated using factors that include demographics, laboratory values and clinical evaluations. We define high-, intermediate-, and low-risk SCA as >15%, 10–15% and <10% 2-year mortality. In walk-PHaSST, no single factor qualifies as high-risk SCA, although several combinations of two factors (that is, both age >35 years and history of chronic transfusion) do. Either elevated white blood cell count (>13.5 × 103 cells/mcL, 7/70 deaths) or elevated Tricuspid Regurgitant Jet Velocity (⩾3.0 m/s, 8/67 deaths) was individually associated with intermediate-risk disease, as were many two-factor combinations. N-terminal pro-brain natriuretic peptide >160 ng/L, lactate dehydrogenase >600 IU/L, history of chronic transfusion, sepsis or age >35 years are individually associated with low-risk SCA, as are many two-factor combinations. SCA risk was integrated with estimated donor type-associated risk from HSCT to form 'Traffic Light' eligibility criteria for clinical trials of HSCT. This method is adaptable to evolutions in clinical care.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lorey FW, Arnopp J, Cunningham GC . Distribution of hemoglobinopathy variants by ethnicity in a multiethnic state. Genet Epidemiol 1996; 13: 501–512.
Johnson FL, Look AT, Gockerman J, Ruggiero MR, Dalla-Pozza L, Billings FT III . Bone-marrow transplantation in a patient with sickle-cell anemia. N Engl J Med 1984; 311: 780–783.
Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood 2007; 110: 2749–2756.
Hsieh M, Courtney F, Weitzel RP, Link M, Wynona C, Xiongce Z et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA 2014; 312: 48–56.
Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 2009; 361: 2309–2317.
Walters M, Patience M, Leisenring W, Eckman J, Buchanan G, Rogers Z et al. Barriers to bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant 1996; 2: 100–104.
Bolaños-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood 2012; 120: 4285–4291.
Quinn CT, Rogers ZR, McCavit TL, Buchanan GR . Improved survival of children and adolescents with sickle cell disease. Blood 2010; 115: 3447–3452.
Abboud MR . Hematopoietic stem-cell transplantation for adults with sickle cell disease. N Engl J Med 2009; 361: 2380–2381.
Angelucci E, Matthes-Martin S, Baronciani D, Bernaudin F, Bonanomi S, Cappellini MD et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica 2014; 99: 811–820.
Shenoy S . Has stem cell transplantation come of age in the treatment of sickle cell disease? Bone Marrow Transplant 2007; 40: 813–821.
Hsieh MM, Fitzhugh CD, Tisdale JF . Allogeneic hematopoietic stem cell transplantation for sickle cell disease: the time is now. Blood 2011; 118: 1197–1207.
Krishnamurti L. Bone Marrow Transplantation in Young Adults With Severe Sickle Cell Disease (STRIDE) (Internet). 2014 (cited 10 May 2013). Available from: http://clinicaltrials.gov/ct2/show/record/NCT01565616.
Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood 2011; 118: 855–864.
Gladwin MT, Barst RJ, Gibbs JSR, Hildesheim M, Sachdev V, Nouraie M et al. Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PLoS One 2014; 9: e99489.
Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH et al. Mortality in sickle cell disease-life expectancy and risk factors for early death. N Engl J Med 1994; 330: 1639–1644.
Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 2004; 350: 886–895.
Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol 2006; 134: 109–115.
De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ . Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcomes. Am J Hematol 2008; 83: 19–25.
Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA 2006; 296: 310–318.
Machado RF, Hildesheim M, Mendelsohn L, Remaley AT, Kato GJ, Gladwin MT . NT-pro brain natriuretic peptide levels and the risk of death in the cooperative study of sickle cell disease. Br J Haematol 2011; 154: 512–520.
Kato GJ, McGowan V, Machado RF, Little JA, Taylor J, Morris CR et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 2006; 107: 2279–2285.
Ayyappan S, Drawz P, Nouraie M, Hildesheim ME, Zhang Y, Gordeuk VR et al. Renal disease in sickle cell: clinically varied and associated with increased mortality. Blood (ASH Annual Meeting Abstracts) 2012; 120: 90.
Ballas SK . Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Semin Hematol 2001, p 30–36.
Sebastiani P, Nolan VG, Baldwin CT, Abad-Grau MM, Wang L, Adewoye AH et al. A network model to predict the risk of death in sickle cell disease. Blood 2007; 110: 2727–2735.
Gardner K, Suddle A, Kane P, O’Grady J, Heaton N, Bomford A et al. How we treat sickle hepatopathy and liver transplantation in adults. Blood 2014; 123: 2302–2307.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999; 130: 461–470.
Gladwin MT, Vichinsky E . Pulmonary complications of sickle cell disease. N Engl J Med 2008; 359: 2254–2265.
Sebastiani P. On line calculator of severity of sickle cell disease (Internet) (cited 30 Jan 2012). Available from: http://www.bu.edu/sicklecell/projects/.
Vermylen C, Cornu G, Ferster A, Brichard B, Ninane J, Ferrant A et al. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplant 1998; 22: 1–6.
Walters MC, Storb R, Patience M, Leisenring W, Taylor T, Sanders JE et al. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Blood 2000; 95: 1918–1924.
Van Besien K, Koshy M, Anderson-Shaw L, Talishy N, Dorn L, Devine S et al. Allogeneic stem cell transplantation for sickle cell disease. A study of patients’ decisions. Bone marrow transplant 2001; 28: 545–549.
Kodish E, Lantos J, Stocking C, Singer PA, Siegler M, Johnson FL . Bone marrow transplantation for sickle cell disease: a study of parents’ decisions. N Engl J Med 1991; 325: 1349–1353.
Chakrabarti S, Bareford D . A survey on patient perception of reduced-intensity transplantation in adults with sickle cell disease. Bone Marrow Transplant 2007; 39: 447–451.
Zhang M-J, Davies SM, Camitta BM, Logan B, Tiedemann K, Eapen M . Comparison of outcomes after HLA-matched sibling and unrelated donor transplantation for children with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2012; 18: 1204–1210.
Shenoy S, Grossman W, DiPersio J, Yu L, Wilson D, Barnes Y et al. A novel reduced-intensity stem cell transplant regimen for nonmalignant disorders. Bone Marrow Transplant 2005; 35: 345–352.
Ruggeri A, Eapen M, Scaravadou A, Cairo MS, Bhatia M, Kurtzberg J et al. Umbilical cord blood transplantation for children with thalassemia and sickle cell disease. Biol Blood Marrow Transplant 2011; 17: 1375–1382.
Naik RP, Streiff MB, Haywood C Jr, Nelson JA, Lanzkron S . Venous thromboembolism in adults with sickle cell disease: a serious and under-recognized complication. Am J Med 2013; 126: 443–449.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Gladwin reports the following conflict of interest. Bayer Pharma AG: consultancy and research funding; US Government: co-inventor on a provisional patent for nitrite salts for cardiovascular indications. The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rotz, S., O'Riordan, M., Kim, C. et al. Traffic Light: prognosis-based eligibility for clinical trials of hematopoietic SCT in adults with sickle cell anemia. Bone Marrow Transplant 50, 918–923 (2015). https://doi.org/10.1038/bmt.2015.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.11
This article is cited by
-
Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation
Journal of Thrombosis and Thrombolysis (2021)
-
Allogenic peripheral stem cell transplantation from HLA-matched related donors for adult sickle cell disease: remarkable outcomes from a single-center trial
Bone Marrow Transplantation (2018)
-
Sickle cell disease is not so benign
Bone Marrow Transplantation (2015)