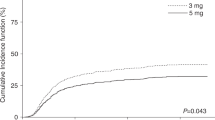
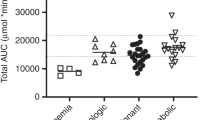
Abstract
Given age-related differences in drug metabolism and indications for hematopoietic SCT (HSCT), personalized drug dosing of the conditioning regimen and post-transplant immunosuppression may reduce graft rejection, relapse rates and toxicity in pediatric HSCT recipients. This manuscript summarizes the pharmacokinetic/dynamic data of HSCT conditioning and post-grafting immunosuppression, presented at the First Annual Pediatric Bone Marrow Transplant Consortium (PBMTC) meeting in April 2013. Personalized dosing of BU to a target plasma exposure reduces graft rejection in children and improves relapse/toxicity rates in adults. Current weight-based dosing achieves the target BU exposure in only a minority (24.3%) of children. The initial BU dose should be based on the European Medicines Agency nomogram or population pharmacokinetic models to improve the numbers of children achieving the target exposure. There are limited pharmacokinetic data for treosulfan, CY, fludarabine and alemtuzumab as HSCT conditioning in children. For post-grafting immunosuppression, mycophenolic acid (MPA) clearance may be increased in younger children (<12 years). The preferred MPA pharmacokinetic monitoring parameters and target range are still evolving in HSCT recipients. Multi-institutional trials incorporating properly powered pharmacokinetic/dynamic studies are needed to assess the effect of variability in the plasma exposure of drugs/metabolites on clinical outcomes in pediatric HSCT recipients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Deeg HJ, Maris MB, Scott BL, Warren EH . Optimization of allogeneic transplant conditioning: not the time for dogma. Leukemia 2006; 20: 1701–1705.
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE . Developmental pharmacology-drug disposition, action, and therapy in infants and children. N Engl J Med 2003; 349: 1157–1167.
Woodahl EL, Wang J, Heimfeld S, Sandmaier BM, O'Donnell PV, Phillips B et al. A novel phenotypic method to determine fludarabine triphosphate accumulation in T-lymphocytes from hematopoietic cell transplantation patients. Cancer Chemother Pharmacol 2009; 63: 391–401.
Cinader B . Aging and the Immune System. in: Delves PD, Roitt IM (eds). Encyclopedia of Immunology. San Diego, CA, USA: Academic Press, 1998.
Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy [see comments]. N Engl J Med 1995; 332: 143–149.
Savage WJ, Bleesing JJ, Douek D, Brown MR, Linton GM, Malech HL et al. Lymphocyte reconstitution following non-myeloablative hematopoietic stem cell transplantation follows two patterns depending on age and donor/recipient chimerism. Bone Marrow Transplant 2001; 28: 463–471.
Newborn Screening for Severe Combined Immunodeficiency Disorder. Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children Report. Accessed October 3rd, 2014 at http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/combinedimmunodeficiency.pdf).
Centers for Disease Control and Prevention (CDC). Impact of expanded newborn screening-United States, 2006. MMWR Morb Mortal Wkly Rep 2008; 57: 1012–1015.
Angiolillo AL, Yu AL, Reaman G, Ingle AM, Secola R, Adamson PC . A phase II study of Campath-1H in children with relapsed or refractory acute lymphoblastic leukemia: a children’s oncology group report. Pediatr Blood Cancer 2009; 53: 978–983.
Huezo-Diaz P, Uppugunduri CR, Tyagi AK, Krajinovic M, Ansari M . Pharmacogenetic aspects of drug metabolizing enzymes in busulfan based conditioning prior to allogenic hematopoietic stem cell transplantation in children. Curr Drug Metab 2014; 15: 251–264.
Ten Brink MH, Zwaveling J, Swen JJ, Bredius RG, Lankester AC, Guchelaar HJ . Personalized busulfan and treosulfan conditioning for pediatric stem cell transplantation: the role of pharmacogenetics and pharmacokinetics. Drug Discov Today 2014; 19: 1572–1586.
Kletzel M, Jacobsohn D, Duerst R . Pharmacokinetics of a test dose of intravenous busulfan guide dose modifications to achieve an optimal area under the curve of a single daily dose of intravenous busulfan in children undergoing a reduced-intensity conditioning regimen with hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2006; 12: 472–479.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
Kroger N, Schetelig J, Zabelina T, Kruger W, Renges H, Stute N et al. A fludarabine-based dose-reduced conditioning regimen followed by allogeneic stem cell transplantation from related or unrelated donors in patients with myelodysplastic syndrome. Bone Marrow Transplant 2001; 28: 643–647.
Ho AYL, Pagliuca A, Kenyon M, Parker JE, Mijovic A, Devereux S et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzumab (FBC) conditioning. Blood 2004; 104: 1616–1623.
Mohty M, Bay J-O, Faucher C, Choufi B, Bilger K, Tournilhac O et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood 2003; 102: 470–476.
Kahl CA, Tarantal AF, Lee CI, Jimenez DF, Choi C, Pepper K et al. Effects of busulfan dose escalation on engraftment of infant rhesus monkey hematopoietic stem cells after gene marking by a lentiviral vector. Exp Hematol 2006; 34: 369–381.
Tarantal AF, Giannoni F, Lee CC, Wherley J, Sumiyoshi T, Martinez M et al. Nonmyeloablative conditioning regimen to increase engraftment of gene-modified hematopoietic stem cells in young rhesus monkeys. Mol Ther 2012; 20: 1033–1045.
Candotti F, Shaw KL, Muul L, Carbonaro D, Sokolic R, Choi C et al. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans. Blood 2012; 120: 3635–3646.
Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002; 296: 2410–2413.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006; 12: 401–409.
Nieder ML, McDonald GB, Kida A, Hingorani S, Armenian SH, Cooke KR et al. National Cancer Institute-National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: long-term organ damage and dysfunction. Biol Blood Marrow Transplant 2011; 17: 1573–1584.
McCune JS, Holmberg LA . Busulfan in hematopoietic stem cell transplant setting. Expert Opin Drug Metab Toxicol 2009; 5: 957–969.
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 1995; 16: 31–42.
Bolinger AM, Zangwill AB, Slattery JT, Glidden D, DeSantes K, Heyn L et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone marrow transplant 2000; 25: 925–930.
Bolinger AM, Zangwill AB, Slattery JT, Risler LJ, Sultan DH, Glidden DV et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1013–1018.
Busulfex IV Product Informaiton. In: Otsuka Pharmaceuticals America I (ed.) 2011.
Bredeson C . Intravenous versus oral busulfan-based conditioning for pediatric allogeneic hematopoietic cell transplantations: did the pendulum swing too far, too fast? Biol Blood Marrow Transplant 2013; 19: 1657–1658.
Kato M, Takahashi Y, Tomizawa D, Okamoto Y, Inagaki J, Koh K et al. Comparison of intravenous with oral busulfan in allogeneic hematopoietic stem cell transplantation with myeloablative conditioning regimens for pediatric acute leukemia. Biol Blood Marrow Transplant 2013; 19: 1690–1694.
Gordon N, Mullen CA, Tran H, Worth L, Gomez Almaguer D, Chan KW . Fludarabine and once-daily intravenous busulfan for allogeneic bone marrow transplantation for Chediak-Higashi syndrome. J Pediatr Hematol Oncol 2003; 25: 824–826.
Lee JW, Kang HJ, Lee SH, Yu KS, Kim NH, Yuk YJ et al. Highly variable pharmacokinetics of once-daily intravenous busulfan when combined with fludarabine in pediatric patients: phase I clinical study for determination of optimal once-daily busulfan dose using pharmacokinetic modeling. Biol Blood Marrow Transplant 2012; 18: 944–950.
Tse WT, Duerst R, Schneiderman J, Chaudhury S, Jacobsohn D, Kletzel M . Age-dependent pharmacokinetic profile of single daily dose i.v. busulfan in children undergoing reduced-intensity conditioning stem cell transplant. Bone Marrow Transplant 2009; 44: 145–156.
Zwaveling J, den Hartigh J, Lankester AC, Guchelaar HJ, Egeler RM, Bredius RG . Once-daily intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Anticancer Drugs 2006; 17: 1099–1105.
McCune JS, Baker KS, Blough DK, Gamis A, Bemer MJ, Kelton-Rehkopf MC et al. Variation in Prescribing Patterns and Therapeutic Drug Monitoring of Intravenous Busulfan in Pediatric Hematopoietic Cell Transplant Recipients. J Clin Pharmacol 2013; 53: 264–275.
McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH . Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and bayesian dose personalization. Clin Cancer Res 2014; 20: 754–763.
Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function [see comments]. J Clin Oncol 1989; 7: 1748–1756.
Nguyen L, Fuller D, Lennon S, Leger F, Puozzo C . I.V. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant 2004; 33: 979–987.
McCune JS, Gibbs JP, Slattery JT . Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet 2000; 39: 155–165.
McCune JS, Gooley T, Gibbs JP, Sanders JE, Petersdorf EW, Appelbaum FR et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 30: 167–173.
Meresse V, Hartmann O, Vassal G, Benhamou E, Valteau-Couanet D, Brugieres L et al. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant 1992; 10: 135–141.
Zwaveling J, Bredius RG, Cremers SC, Ball LM, Lankester AC, Teepe-Twiss IM et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant 2005; 35: 17–23.
Baker KS, Bostrom B, DeFor T, Ramsay NK, Woods WG, Blazar BR . Busulfan pharmacokinetics do not predict relapse in acute myeloid leukemia. Bone Marrow Transplant 2000; 26: 607–614.
Slatter MA, Rao K, Amrolia P, Flood T, Abinun M, Hambleton S et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood 2011; 117: 4367–4375.
Bernardo ME, Piras E, Vacca A, Giorgiani G, Zecca M, Bertaina A et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood 2012; 120: 473–476.
Glowka FK, Karazniewicz-Lada M, Grund G, Wrobel T, Wachowiak J . Pharmacokinetics of high-dose i.v. treosulfan in children undergoing treosulfan-based preparative regimen for allogeneic haematopoietic SCT. Bone Marrow Transplant 2008; 42 (Suppl 2): S67–S70.
Treosulfan/Fludarabine/Low Dose TBI as a Preparative Regimen for Children With AML/MDS Undergoing Allo HCT Clinicaltrials.gov Identifier: NCT01772953 (Co-PIs: Eneida Nemecek and Colleen Delaney). (Accessed December 4, 2013, at http://clinicaltrials.gov/ct2/show/NCT01772953?term=treosulfan&age=0&rank=1).
Ten Brink MH, Ackaert O, Zwaveling J, Bredius RG, Smiers FJ, den Hartigh J et al. Pharmacokinetics of treosulfan in pediatric patients undergoing hematopoietic stem cell transplantation. Ther Drug Monit 2014; 36: 465–472.
de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH . Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 2005; 44: 1135–1164.
McCune JS, Salinger DH, Vicini P, Oglesby C, Blough DK, Park JR . Population pharmacokinetics of cyclophosphamide and metabolites in children with neuroblastoma: a report from the children's oncology group. J Clin Pharmacol 2009; 49: 88–102.
Balasubramanian P, Desire S, Panetta JC, Lakshmi KM, Mathews V, George B et al. Population pharmacokinetics of cyclophosphamide in patients with thalassemia major undergoing HSCT. Bone Marrow Transplant 2012; 47: 1178–1185.
Qiu R, Yao A, Vicini P, McDonald GB, Batchelder AL, Bouvier ME et al. Diminishing the risk of nonrelapse mortality in hematopoietic stem cell transplantation: Prediction of exposure to the cyclophosphamide metabolite carboxyethylphosphoramide mustard. Clin Pharmacol Ther 2004; 76: 270–280.
Raccor BS, Claessens AJ, Dinh JC, Park JR, Hawkins DS, Thomas SS et al. Potential contribution of cytochrome P450 2B6 to hepatic 4-hydroxycyclophosphamide formation in vitro and in vivo. Drug Metab Dispos 2012; 40: 54–63.
McCune JS, Batchelder A, Deeg HJ, Gooley T, Cole S, Phillips B et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant 2007; 13: 853–862.
Danhauser L, Plunkett W, Liliemark J, Gandhi V, Iacoboni S, Keating M . Comparison between the plasma and intracellular pharmacology of 1-beta-D-arabinofuranosylcytosine and 9-beta-D-arabinofuranosyl-2-fluoroadenine 5'-monophosphate in patients with relapsed leukemia. Leukemia 1987; 1: 638–643.
Gandhi V, Plunkett W . Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet 2002; 41: 93–103.
Robak T, Lech-Maranda E, Korycka A, Robak E . Purine nucleoside analogs as immunosuppressive and antineoplastic agents: mechanism of action and clinical activity. Curr Med Chem 2006; 13: 3165–3189.
Salinger DH, Blough DK, Vicini P, Anasetti C, O'Donnell PV, Sandmaier BM et al. A limited sampling schedule to estimate individual pharmacokinetic parameters of fludarabine in hematopoietic cell transplant patients. Clin Cancer Res 2009; 15: 5280–5287.
Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood 2003; 102: 820–826.
Jacobson PA, Rogosheske J, Green K, Brunstein C, Barker J, Miller J et al. Fludarabine pharmacokinetics in nonmyeloablative hematopoietic cell transplantation (HCT): association with engraftment and neurotoxicty. Blood 2005; 106: Abstract #3673.
Law J, Cowan MJ, Dvorak CC, Musick L, Long-Boyle JR, Baxter-Lowe LA et al. Busulfan, fludarabine, and alemtuzumab as a reduced toxicity regimen for children with malignant and nonmalignant diseases improves engraftment and graft-versus-host disease without delaying immune reconstitution. Biol Blood Marrow Transplant 2012; 18: 1656–1663.
Styczynski J, Tallamy B, Waxman I, van de Ven C, Milone MC, Shaw LM et al. A pilot study of reduced toxicity conditioning with BU, fludarabine and alemtuzumab before the allogeneic hematopoietic SCT in children and adolescents. Bone Marrow Transplant 2011; 46: 790–799.
Spyridonidis A, Liga M, Triantafyllou E, Themeli M, Marangos M, Karakantza M et al. Pharmacokinetics and clinical activity of very low-dose alemtuzumab in transplantation for acute leukemia. Bone Marrow Transplant 2011; 46: 1363–1368.
Elter T, Molnar I, Kuhlmann J, Hallek M, Wendtner C . Pharmacokinetics of alemtuzumab and the relevance in clinical practice. Leuk Lymphoma 2008; 49: 2256–2262.
Mould DR, Baumann A, Kuhlmann J, Keating MJ, Weitman S, Hillmen P et al. Population pharmacokinetics-pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br J Clin Pharmacol 2007; 64: 278–291.
Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldstone AH et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood 2003; 102: 404–406.
Rebello P, Cwynarski K, Varughese M, Eades A, Apperley JF, Hale G . Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy 2001; 3: 261–267.
Li H, Mager DE, Sandmaier BM, Maloney DG, Bemer MJ, McCune JS . Population pharmacokinetics and dose optimization of mycophenolic acid in HCT recipients receiving oral mycophenolate mofetil. J Clin Pharmacol 2013; 53: 393–402.
Frymoyer A, Verotta D, Jacobson P, Long-Boyle J . Population pharmacokinetics of unbound mycophenolic acid in adult allogeneic haematopoietic cell transplantation: effect of pharmacogenetic factors. Br J Clin Pharmacol 2013; 75: 463–475.
Jacobson P, Green K, Rogosheske J, Brunstein C, Ebeling B, DeFor T et al. Highly variable mycophenolate mofetil bioavailability following nonmyeloablative hematopoietic cell transplantation. J Clin Pharmacol 2007; 47: 6–12.
Nash RA, Johnston L, Parker P, McCune JS, Storer B, Slattery JT et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2005; 11: 495–505.
Rupprecht K, Schmidt C, Raspe A, Schweda F, Shipkova M, Fischer W et al. Bioavailability of mycophenolate mofetil and enteric-coated mycophenolate sodium is differentially affected by pantoprazole in healthy volunteers. J Clin Pharmacol 2009; 49: 1196–1201.
Borrows R, Chusney G, Loucaidou M, James A, Van Tromp J, Cairns T et al. The magnitude and time course of changes in mycophenolic acid 12-hour predose levels during antibiotic therapy in mycophenolate mofetil-based renal transplantation. Ther Drug Monit 2007; 29: 122–126.
Naito T, Shinno K, Maeda T, Kagawa Y, Hashimoto H, Otsuka A et al. Effects of calcineurin inhibitors on pharmacokinetics of mycophenolic acid and its glucuronide metabolite during the maintenance period following renal transplantation. Biol Pharm Bull 2006; 29: 275–280.
Hesselink DA, van Hest RM, Mathot RA, Bonthuis F, Weimar W, de Bruin RW et al. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am J Transplant 2005; 5: 987–994.
Zucker K, Rosen A, Tsaroucha A, de Faria L, Roth D, Ciancio G et al. Unexpected augmentation of mycophenolic acid pharmacokinetics in renal transplant patients receiving tacrolimus and mycophenolate mofetil in combination therapy, and analogous in vitro findings. Transpl Immunol 1997; 5: 225–232.
de Winter BC, Mathot RA, Sombogaard F, Vulto AG, van Gelder T . Nonlinear relationship between mycophenolate mofetil dose and mycophenolic acid exposure: implications for therapeutic drug monitoring. Clin J Am Soc Nephrol 2011; 6: 656–663.
Huang J, Jacobson P, Brundage R . Prediction of unbound mycophenolic acid concentrations in patients after hematopoietic cell transplantation. Ther Drug Monit 2007; 29: 385–390.
Jacobson P, Long J, Rogosheske J, Brunstein C, Weisdorf D . High unbound mycophenolic acid concentrations in a hematopoietic cell transplantation patient with sepsis and renal and hepatic dysfunction. Biol Blood Marrow Transplant 2005; 11: 977–978.
Jacobson PA, Rydhom N, Huang J, Baker KS, Verneris MR . High-unbound mycophenolic acid concentrations in an infant on peritoneal dialysis following hematopoietic cell transplant. Bone Marrow Transplant 2007; 40: 911–912.
Kim H, Long-Boyle J, Rydholm N, Orchard PJ, Tolar J, Smith AR et al. Population pharmacokinetics of unbound mycophenolic acid in pediatric and young adult patients undergoing allogeneic hematopoietic cell transplantation. J Clin Pharmacol 2012; 52: 1665–1675.
de Winter BC, Mathot RA, Sombogaard F, Neumann I, van Hest RM, Doorduijn JK et al. Differences in clearance of mycophenolic acid among renal transplant recipients, hematopoietic stem cell transplant recipients, and patients with autoimmune disease. Ther Drug Monit 2010; 32: 606–614.
Jacobson P, Rogosheske J, Barker JN, Green K, Ng J, Weisdorf D et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther 2005; 78: 486–500.
Jacobson P, Huang J, Rydholm N, Tran M, Defor T, Tolar J et al. Higher mycophenolate dose requirements in children undergoing hematopoietic cell transplant (HCT). J Clin Pharmacol 2008; 48: 485–494.
Giaccone L, McCune JS, Maris MB, Gooley TA, Sandmaier BM, Slattery JT et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood 2005; 106: 4381–4388.
McDermott CL, Sandmaier BM, Storer B, Li H, Mager DE, Boeckh MJ et al. Nonrelapse mortality and mycophenolic acid exposure in nonmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant 2013; 19: 1159–1166.
Jacobson PA, Huang J, Wu J, Kim M, Logan B, Alousi A et al. Mycophenolate pharmacokinetics and association with response to acute graft vs host disease (GVHD) treatment from the blood and marrow transplant clinical trials network. Biol Blood Marrow Transplant 2010; 16: 421–429.
Jacobson P, El-Massah SF, Rogosheske J, Kerr A, Long-Boyle J, DeFor T et al. Comparison of two mycophenolate mofetil dosing regimens after hematopoietic cell transplantation. Bone Marrow Transplant 2009; 44: 113–120.
Bhatia M, Militano O, Jin Z, Figurski M, Shaw L, Moore V et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant 2010; 16: 333–343.
Gibbs JP, Liacouras CA, Baldassano RN, Slattery JT . Up-regulation of glutathione s-transferase activity in enterocytes of young children. Drug Metab Dispos 1999; 27: 1466–1469.
Booth BP, Rahman A, Dagher R, Griebel D, Lennon S, Fuller D et al. Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J Clin Pharmacol 2007; 47: 101–111.
Jenke A, Renner U, Richte M, Freiberg-Richter J, Platzbecker U, Helwig A et al. Pharmacokinetics of intravenous mycophenolate mofetil after allogeneic blood stem cell transplantation. Clin Transplant 2001; 15: 176–184.
van Hest RM, Doorduijn JK, de Winter BC, Cornelissen JJ, Vulto AG, Oellerich M et al. Pharmacokinetics of mycophenolate mofetil in hematopoietic stem cell transplant recipients. Ther Drug Monit 2007; 29: 353–360.
Haentzschel I, Freiberg-Richter J, Platzbecker U, Kiani A, Schetelig J, Illmer T et al. Targeting mycophenolate mofetil for graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Bone Marrow Transplant 2008; 42: 113–120.
Acknowledgements
This work was supported by grants from the NCI (#1R13CA177155-01, 1R01CA182963, 1R21162059), NHLBI (#5U10HL069254-13), Pediatric Cancer Research Foundation, Children’s Cancer Fund, NMDP Foundation and St Baldrick’s Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr McCune has received research funding from Otsuka Pharmaceutical of North America. All other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
McCune, J., Jacobson, P., Wiseman, A. et al. Optimizing drug therapy in pediatric SCT: Focus on pharmacokinetics. Bone Marrow Transplant 50, 165–172 (2015). https://doi.org/10.1038/bmt.2014.235
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.235
This article is cited by
-
Pharmacokinetics of fludarabine and its association with clinical outcomes in paediatric haematopoietic stem cell transplantation patients
Bone Marrow Transplantation (2019)
-
Clinical Pharmacokinetics of Mycophenolic Acid in Hematopoietic Stem Cell Transplantation Recipients
European Journal of Drug Metabolism and Pharmacokinetics (2017)
-
Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect
Nature Reviews Clinical Oncology (2016)
-
Pharmacokinetics, Pharmacodynamics, and Pharmacogenomics of Immunosuppressants in Allogeneic Hematopoietic Cell Transplantation: Part II
Clinical Pharmacokinetics (2016)