Abstract
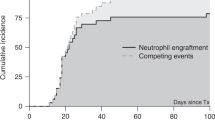
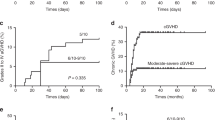
Haploidentical hematopoietic SCT (haplo-HSCT) is to be established in patients with acquired severe aplastic anemia (SAA) refractory to immunosuppressive therapy and lacking HLA-matched related or unrelated donors. Graft failure (GF) and GVHD have been major obstacles to HSCT. A total of 17 children and adolescents with SAA underwent haplo-HSCT in our center. The conditioning regimen consisted of BU, fludarabine, CY and anti-thymocyte globulin. All patients received cyclosporine, short-term MTX, mycophenolate mofetil and basiliximab for GVHD prophylaxis. Mesenchymal stem cells derived from unrelated umbilical cord were infused on day 1. Neutrophil engraftment was achieved in all 17 patients in a median time of 16 days (range 9–25 days). The median time of platelet engraftment was 22 days (range 9–95 days) in 16 patients. The cumulative incidence (CI) of II–IV acute GVHD (aGVHD) at day +100 was 30.53±11.12% and III–IV aGVHD occurred in only one patient. The CI of chronic GVHD was 21.25±13.31%. Secondary GF with autologous hematopoiesis recovery occurred in one patient. The OS was 71.60±17.00% at a median follow-up of 362 (36–1321) days. These limited promising data suggest that haplo-HSCT is feasible as a salvage therapy for children and adolescents with refractory SAA who lack matched donors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Young NS, Calado RT, Scheinberg P . Current concepts in the pathophysiology and treatment of aplastic anemia. Blood 2006; 108: 2509–2519.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107: 3065–3073.
Passweg JR, Pérez WS, Eapen M, Camitta BM, Gluckman E, Hinterberger W et al. Bone marrow transplants from mismatched related and unrelated donors for severe aplastic anemia. Bone Marrow Transplant 2006; 37: 641–649.
Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant 2012; 47: 1507–1512.
Wang Z, Yan H, Zhu L, Wang H . Successful unmanipulated stem cell transplantation from HLA-haploidentical 3-loci-mismatched parents in two children with severe aplastic anemia not responding to immunosuppressive therapy. Am J Hematol 2010; 85: 389–390.
Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood 1976; 48: 63–70.
Wang HX, Wang ZD, Zheng XL, Ding L, Zhu L, Yan HM et al. Hematopoietic stem cell transplantation with umbilical cord stromal cell infusion for the treatment of aplastic anemia—a single-center experience. Cytotherapy 2013; 15: 1118–1125.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–1568.
Gupta V, Eapen M, Brazauskas R, Carreras J, Aljurf M, Gale RP et al. Impact of age on outcomes after bone marrow transplantation for acquired aplastic anemia using HLA-matched sibling donors. Haematologica 2010; 95: 2119–2125.
Dong L, Wu T, Gao ZY, Zhang MJ, Kan F, Spellman SR et al. The outcomes of family haploidentical hematopoietic stem cell transplantation in hematologic malignancies are not associated with patient age. Biol Blood Marrow Transplant 2011; 17: 1205–1213.
Wagner JL, Deeg HJ, Seidel K, Anasetti C, Doney K, Sanders J et al. Bone marrow transplantation for severe aplastic anemia from genotypically HLA-nonidentical relatives: an update of the Seattle experience. Transplantation 1996; 61: 54–61.
Tzeng CH, Chen PM, Fan S, Liu JH, Chiou TJ, Hsieh RK . CY/TBI-800 as a pretransplant regimen for allogeneic bone marrow transplantation for severe aplastic anemia using HLA-haploidentical family donors. Bone Marrow Transplant 1996; 18: 273–277.
Maury S, Bacigalupo A, Anderlini P, Aljurf M, Marsh J, Socié G et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica 2009; 94: 1312–1315.
Kang HJ, Shin HY, Park JE, Chung NG, Cho B, Kim HK et al. Successful engraftment with fludarabine, cyclophosphamide, and thymoglobulin conditioning regimen in unrelated transplantation for severe aplastic anemia: A phase II prospective multicenter study. Biol Blood Marrow Transplant 2010; 16: 1582–1588.
Dulley FL, Vigorito AC, Aranha FJ, Sturaro D, Ruiz MA, Saboya R et al. Addition of low-dose busulfan to cyclophosphamide in aplastic anemia patients prior to allogeneic bone marrow transplantation to reduce rejection. Bone Marrow Transplant 2004; 33: 9–13.
Serody JS, Sparks SD, Lin Y, Capel EJ, Bigelow SH, Kirby SL et al. Comparison of granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood progenitor cells and G-CSF–stimulated bone marrow as a source of stem cells in HLA-matched sibling transplantation. Biol Blood Marrow Transplant 2000; 6: 434–440.
Ji SQ, Chen HR, Wang HX, Yan HM, Zhu L, Liu J et al. G-CSF-primed haploidentical marrow transplantation without ex vivo T cell depletion: an excellent alternative for high-risk leukemia. Bone Marrow Transplant 2002; 30: 861–866.
Huang XJ, Chang YJ, Zhao XY . Maintaining hyporesponsiveness and polarization potential of T cells after in vitro mixture of G-CSF mobilized peripheral blood grafts and G-CSF primed bone marrow grafts in different proportions. Transpl Immunol 2007; 17: 193–197.
Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant 2004; 33: 597–604.
Ji SQ, Chen HR, Yan HM, Wang HX, Liu J, Zhu PY et al. Anti-CD25 monoclonal antibody (basiliximab) for prevention of graft-versus-host disease after haploidentical bone marrow transplantation for hematological malignancies. Bone Marrow Transplant 2005; 36: 349–354.
Fang J, Hu C, Hong M, Wu Q, You Y, Zhong Z et al. Prophylactic effects of interleukin-2 receptor antagonists against graft-versus-host disease following unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 754–762.
Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007; 110: 2764–2767.
Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L et al. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant 2010; 16: 838–847.
Acknowledgements
This work was supported by Grant No. 81241016 from the NSFC in China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Z., Zheng, X., Yan, H. et al. Good outcome of haploidentical hematopoietic SCT as a salvage therapy in children and adolescents with acquired severe aplastic anemia. Bone Marrow Transplant 49, 1481–1485 (2014). https://doi.org/10.1038/bmt.2014.187
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.187
This article is cited by
-
Mesenchymal stromal cells as prophylaxis for graft-versus-host disease in haplo-identical hematopoietic stem cell transplantation recipients with severe aplastic anemia?—a systematic review and meta-analysis
Stem Cell Research & Therapy (2021)
-
Immunosuppressive therapy versus haploidentical transplantation in adults with acquired severe aplastic anemia
Bone Marrow Transplantation (2019)
-
Update of the “Beijing Protocol” haplo-identical hematopoietic stem cell transplantation
Bone Marrow Transplantation (2019)
-
Donor-derived marrow mesenchymal stromal cell co-transplantation following a haploidentical hematopoietic stem cell transplantation trail to treat severe aplastic anemia in children
Annals of Hematology (2019)
-
Unmanipulated haploidentical transplantation conditioning with busulfan, cyclophosphamide and anti-thymoglobulin for adult severe aplastic anaemia
Bone Marrow Transplantation (2018)