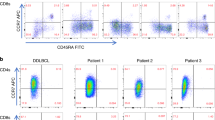
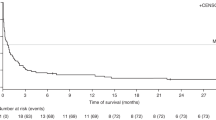
Abstract
EBV-induced post transplantation lymphoproliferative disorder (EBV-PTLD) is a life-threatening complication after allogeneic hematopoietic cell transplantation. Profound T-cell depletion of the allograft represents a major risk factor for EBV-PTLD. With regard to the increasing use of alternative stem cell sources such as cord blood or purified haploidentical stem cell grafts both associated with impaired immune reconstitution, the frequent occurrence of EBV-PTLD demands particular vigilance on laboratory changes and early symptoms. Here we have summarized today’s knowledge about EBV-PTLD in a comprehensive review explaining the underlying mechanisms of EBV-based transformation, EBV-PTLD development, clinical presentation, incidence, diagnosis, screening, therapy and prognosis. In this context, we emphasize on the necessity of regularly applied screening tools and pre-emptive treatment strategies including anti-CD20 Abs particularly in high-risk patients to avoid disease progression to malignant lymphoma. Although EBV-PTLD has always been associated with a high mortality rate, novel immunotherapeutic approaches such as the transfer of EBV-specific T cells nowadays offer improved chances of disease control even at late stages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ruf S, Moser O, Wössmann W, Kreyenberg H, Wagner HJ . Examining the origin of posttransplant lymphoproliferative disorder in a patient after a second allogeneic hematopoeitic stem cell transplantation for relapsed BCR-ABL positive acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2011; 33: 50–54.
Reddiconto G, Chiusolo P, Fiorini A, Farina G, Laurenti L, Martini M et al. Assessment of cellular origin and EBV status in a PTLD after double cord blood transplantation. Leukemia 2007; 21: 2552–2554.
Gong JZ, Bayerl MG, Sandhaus LM, Sebastian S, Rehder CW, Routbort M et al. Posttransplant lymphoproliferative disorder after umbilical cord blood transplantation in children. Am J Surg Pathol 2006; 30: 328–336.
Johansson JE, Remberger M, Lazarevic VLj, Hallböök H, Wahlin A, Kimby E et al. Allogeneic haematopoietic stem-cell transplantation with reduced intensity conditioning for advanced stage Hodgkin’s lymphoma in Sweden: high incidence of post transplant lymphoproliferative disorder. Bone Marrow Transplant 2011; 46: 870–875.
Hou HA, Yao M, Tang JL, Chen YK, Ko BS, Huang SY et al. Poor outcome in post transplant lymphoproliferative disorder with pulmonary involvement after allogeneic hematopoietic SCT: 13 years’ experience in a single institute. Bone Marrow Transplant 2009; 43: 315–321.
Buyck HC, Ball S, Junagade P, Marsh J, Chakrabarti S . Prior immunosuppressive therapy with antithymocyte globulin increases the risk of EBV-related lymphoproliferative disorder following allo-SCT for acquired aplastic anaemia. Bone Marrow Transplant 2009; 43: 813–816.
Ocheni S, Kroeger N, Zabelina T, Sobottka I, Ayuk F, Wolschke C et al. EBV reactivation and post transplant lymphoproliferative disorders following allogeneic SCT. Bone Marrow Transplant 2008; 42: 181–186.
Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood 2009; 113: 4992–5001.
Styczynski J, Einsele H, Gil L, Ljungman P . Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis 2009; 11: 383–392.
Omar H, Hägglund H, Gustafsson-Jernberg A, LeBlanc K, Mattsson J, Remberger M et al. Targeted monitoring of patients at high risk of post-transplant lymphoproliferative disease by quantitative Epstein-Barr virus polymerase chain reaction. Transpl Infect Dis 2009; 11: 393–399.
Sundin M, Le Blanc K, Ringdén O, Barkholt L, Omazic B, Lergin C et al. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica 2006; 91: 1059–1067.
Agbalika F, Larghero J, Esperou H, Marais D, Robin M, Foïs E et al. Epstein-Barr virus early-antigen antibodies before allogeneic haematopoietic stem cell transplantation as a marker of risk of post-transplant lymphoproliferative disorders. Br J Haematol 2007; 136: 305–308.
Islam MS, Anoop P, Gordon-Smith EC, Rice P, Datta-Nemdharry P, Marsh JC . Epstein-Barr virus infections after allogeneic stem cell transplantation: a comparison between non-malignant and malignant hematological disorders. Hematology 2010; 15: 344–350.
Carpenter B, Haque T, Dimopoulou M, Atkinson C, Roughton M, Grace S et al. Incidence and dynamics of Epstein-Barr virus reactivation after alemtuzumab-based conditioning for allogeneic hematopoietic stem-cell transplantation. Transplantation 2010; 90: 564–570.
Chiusolo P, Metafuni E, Cattani P, Piccirillo N, Santangelo R, Manzara S et al. Prospective evaluation of epstein-barr virus reactivation after stem cell transplantation: association with monoclonal gammopathy. J Clin Immunol 2010; 30: 894–902.
Cohen JI . Epstein-Barr virus infection. N Engl J Med 2000; 343: 481–492.
Yates J, Warren N, Reisman D, Sugden B . A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci USA 1984; 81: 3806–3810.
Kalinova L, Indrakova J, Bachleda P . Post-transplant lymphoproliferative disorder. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2009; 153: 251–257.
Friedberg JW, Swinnen LJ . Post-Transplant Lymphproliferative Disease in The Lymphomas Second Edition Elsevier: Philadelphia, PA, USA, 2006.
Diekmann J, Adamopoulou E, Beck O, Rauser G, Lurati S, Tenzer S et al. Processing of two latent membrane protein 1 MHC class I epitopes requires tripeptidyl peptidase II involvement. J Immunol 2009; 183: 1587–1597.
Wang D, Liebowitz D, Kieff E, An EBV . membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 1985; 43 (3 Pt 2): 831–840.
Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A . Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA 1993; 90: 8479–8483.
Swerdlow SH, Webber SA, Chadbrun A, Ferry JA . WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC: Lyon, France, 2008.
Knowles DM, Cesarman E, Chadburn A, Frizzera G, Chen J, Rose EA et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood 1995; 85: 552–565.
Comoli P, Basso S, Zecca M, Pagliara D, Baldanti F, Bernardo ME et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant 2007; 7: 1648–1655.
Muramatsu H, Takahashi Y, Shimoyama Y, Doisaki S, Nishio N, Ito Y et al. CD20-negative Epstein-Barr virus-associated post-transplant lymphoproliferative disease refractory to rituximab in a patient with severe aplastic anemia. Int J Hematol 2011; 93: 779–781.
Deeg HJ, Socie G . Malignancies after hematopoietic stem cell transplantation: many questions, some answers. Blood 1998; 91: 1833–1844.
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15: 1143–1238.
Ahmad I, Cau NV, Kwan J, Maaroufi Y, Meuleman N, Aoun M et al. Preemptive management of Epstein-Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation 2009; 87: 1240–1245.
Blaes AH, Cao Q, Wagner JE, Young JA, Weisdorf DJ, Brunstein CG . Monitoring and preemptive rituximab therapy for Epstein-Barr virus reactivation after antithymocyte globulin containing nonmyeloablative conditioning for umbilical cord blood transplantation. Biol Blood Marrow Transplant 2010; 16: 287–291.
Weinstock DM, Ambrossi GG, Brennan C, Kiehn TE, Jakubowski A . Preemptive diagnosis and treatment of Epstein-Barr virus-associated post transplant lymphoproliferative disorder after hematopoietic stem cell transplant: an approach in development. Bone Marrow Transplant 2006; 37: 539–546.
Rowe DT, Webber S, Schauer EM, Reyes J, Green M . Epstein-Barr virus load monitoring: its role in the prevention and management of post-transplant lymphoproliferative disease. Transpl Infect Dis 2001; 3: 79–87.
Meijer E, Cornelissen JJ . Epstein-Barr virus-associated lymphoproliferative disease after allogeneic haematopoietic stem cell transplantation: molecular monitoring and early treatment of high-risk patients. Curr Opin Hematol 2008; 15: 576–585.
Beck R, Westdörp I, Jahn G, Schäfer H, Kanz L, Einsele H . Detection of Epstein-Barr virus DNA in plasma from patients with lymphoproliferative disease after allogeneic bone marrow or peripheral blood stem cell transplantation. J Clin Microbiol 1999; 37: 3430–3431.
McIver Z, Stephens N, Grim A, Barrett AJ . Rituximab administration within 6 months of T cell-depleted allogeneic SCT is associated with prolonged life-threatening cytopenias. Biol Blood Marrow Transplant 2010; 16: 1549–1556.
Stuhler G, Knop S, Topp MS, Kröber SM, Ernemann U, Herrlinger U et al. Intravenously administered rituximab induces remission of EBV associated non Hodgkin lymphoma confined to the brain in a patient after allogeneic stem cell transplantation. Haematologica 2006; 91: ECR01.
Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA . Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010; 115: 925–935.
Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998; 92: 1549–1555.
Moosmann A, Bigalke I, Tischer J, Schirrmann L, Kasten J, Tippmer S et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood 2010; 115: 2960–2970.
Uhlin M, Okas M, Gertow J, Uzunel M, Brismar TB, Mattsson J . A novel haplo-identical adoptive CTL therapy as a treatment for EBV-associated lymphoma after stem cell transplantation. Cancer Immunol Immunother 2010; 59: 473–477.
Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 2010; 116: 5045–5049.
Reisner Y, Hagin D, Martelli MF . Haploidentical hematopoietic transplantation: current status and future perspectives. Blood 2011; 118: 6006–6017.
Mielke S, McIver ZA, Shenoy A, Fellowes V, Khuu H, Stroncek DF et al. Selectively T cell-depleted allografts from HLA-matched sibling donors followed by low-dose posttransplantation immunosuppression to improve transplantation outcome in patients with hematologic malignancies. Biol Blood Marrow Transplant 2011; 17: 1855–1861.
Savani BN, Mielke S, Rezvani K, Montero A, Yong AS, Wish L et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2007; 13: 1216–1223.
Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH et al. Multicenter study of banked third party virus-specific T-cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood, (e-pub ahead of print 22 April 2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rasche, L., Kapp, M., Einsele, H. et al. EBV-induced post transplant lymphoproliferative disorders: a persisting challenge in allogeneic hematopoetic SCT. Bone Marrow Transplant 49, 163–167 (2014). https://doi.org/10.1038/bmt.2013.96
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.96
Keywords
This article is cited by
-
Outcomes for patients with EBV-positive PTLD post-allogeneic HCT after failure of rituximab-containing therapy
Bone Marrow Transplantation (2024)
-
Lack of both donor and recipient anti-EBV T cells in EBV seronegative recipients of grafts from seropositive donors
Bone Marrow Transplantation (2023)
-
Epstein–Barr virus-positive lymphoproliferative disorder manifesting as pulmonary disease in a patient with acute myeloid leukemia: a case report
Journal of Medical Case Reports (2021)
-
Chronic lymphocytic infiltration with pontine perivascular enhancement responsive to steroids (CLIPPERS) and its association with Epstein‐Barr Virus (EBV)-related lymphomatoid granulomatosis: a case report
BMC Neurology (2021)
-
Characteristics of Epstein–Barr virus reactivation after allogeneic haematopoietic stem cell transplantation in patients with chronic active Epstein–Barr virus disease: favorable responses to rituximab
Bone Marrow Transplantation (2021)