Abstract
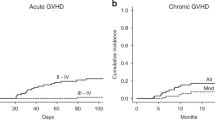
To evaluate the effect of the different doses of antithymocyte globulin (ATG) on the incidence of acute GVHD among patients receiving hematopoietic SCT without ex vivo T-cell-depletion from haploidentical donors, 224 patients with standard-risk hematological malignancy were randomized in this study. One hundred and twelve patients received 6 mg/kg ATG, whereas the remaining patients received 10 mg/kg ATG. This study was registered at http://www.chictr.org as No. ChiCTR-TRC-11001761. The incidence of grade III–IV acute GVHD was higher in the ATG-6 group (16.1%, 95% confidence interval (CI), 9.1–23.1%) than in the ATG-10 group (4.5%, CI, 0.7–8.3%, P=0.005, 95% CI for the difference, −19.4% to −3.8%). EBV reactivation occurred more frequently in the ATG-10 group (25.3%, 17.1–33.5%) than in the ATG-6 group (9.6% (4.0–15.2%), P=0.001). The 1-year disease-free survival rates were 84.3% (77.3–91.3%) and 86.0% (79.2–92.8%) for the ATG-6 group and ATG-10 groups, respectively (P=0.88). In conclusion, although 6 mg/kg ATG applied in haploidentical transplantation decreased the risk of EBV reactivation compared with 10 mg/kg ATG, this treatment exposes patients to a higher risk for severe acute GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Treatment of acute leukaemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant 2009; 15: 257–265.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001; 98: 2942–2947.
Duggan P, Booth K, Chaudhry A, Stewart D, Stewart D, Ruether JD et al. Unrelated donor BMT recipients given pretransplant low-dose antithymocyte globulin have outcomes equivalent to matched sibling BMT: a matched pair analysis. Bone Marrow Transplant 2002; 30: 681–686.
Meijer E, Cornelissen JJ, Lowenberg B, Verdonck LF . Antithymocyteglobulin as prophylaxis of graft failure and graft-versus-host disease in recipients of partially T-cell-depleted grafts from matched unrelated donors: a dose-finding study. Exp Hematol 2003; 31: 1026–1030.
Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W et al. Haploidentical allogeneic hematopoietic stem cell transplantation for the treatment of refractory/relapsed acute leukemia. Chin J Hematol 2012; 33: 916–920.
Huang XJ, Zhu HH, Chang YJ, Xu LP, Liu DH, Zhang XH et al. The superiority of haploidentical related stem cell transplantation over chemotherapy alone as postremission treatment for patients with intermediate- or high-risk acute myeloid leukemia in first complete remission. Blood 2012; 119: 5584–5590.
Yan CH, Liu DH, Xu LP, Xu LP, Liu YR, Chen H et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse and improve survival of patients with standard-risk acute leukemia after allogeneic hematopoietic stem cell transplantation. Blood 2012; 119: 3256–3262.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W . Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haplo-identical T-cell-replete hematopoietic stem cell transplantation. Haematologica 2007; 92: 414–417.
Sullivan KM . Grfte-versus-host-disease. In: Thomas ED, Blume KG, Forman SJ (eds).. Hematopoietic Cell Transplantation 2nd edn. Blackwell Science: Boston, MA, USA, 1999,, pp 515–536.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945.
Russell JA, Turner AR, Larratt L, Chaudhry A, Morris D, Brown C et al. Adult recipients of matched related donor blood cell transplants given myeloablative regimens including pretransplant antithymocyte globulin have lower mortality related to graft -versus-host disease: a matched pair analysis[J]. Biol Blood Marrow Transplant 2007; 13: 299–306.
Christopher N, Zhang M-J, Agovi MA, Bacigalupo A, Bahlis NJ, Ballen K et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide[J]. Biol Blood Marrow Transplant 2008; 14: 993–1003.
Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood 2003; 102: 470–476.
Remberger M, Svahn BM, Mattsson J, Ringden O . Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation 2004; 78: 122–127.
Schleuning M, Gunther W, Tischer J, Ledderose G, Kolb HJ . Dose-dependent effects of in vivo antithymocyte globulin during conditioning for allogeneic bone marrow transplantation from unrelated donors in patients with chronic phase CML. Bone Marrow Transplant 2003; 32: 243–250.
Portier DA, Sabo RT, Roberts CH, Fletcher DS, Meier J, Clark WB et al. Anti-thymocyte globulin for conditioning in matched unrelated donor hematopoietic cell transplantation provides comparable outcomes to matched related donor recipients. Bone Marrow Transplant 2012; 47: 1513–1519.
Reisner Y, Hagin D, Martelli MF . Haploidentical hematopoietic transplantation: current status and future perspectives. Blood 2011; 118: 6006–6017.
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Zhang XH et al. Prevention of relapse using granulocyte CSF-primed PBPCs following HLA-mismatched/haploidentical, T-cell-replete hematopoietic SCT in patients with advanced-stage acute leukemia: a retrospective risk-factor analysis. Bone Marrow Transplant 2012; 47: 1099–1104.
Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant 2012; 47: 1507–1512.
Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in-vitro T-cell-depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 2013; 119: 978–985.
Chao NJ, Snyder DS, Jain M, Wong RM, Niland JC, Negrin RS et al. Equivalence of two effective graft-versus-host disease prophylaxis regimens: results of a prospective double blind randomized trial. Biol Blood Marrow Transplant 2000; 6: 254–261.
Duval M, Pédron B, Rohrlich P, Legrand F, Faye A, Lescoeur B et al. Immune reconstitution after haematopoietic transplantation with two different doses of pre-graft antithymocyte globulin. Bone Marrow Transplant 2002; 30: 421–426.
Ramsay NKC, Kersey JH, Robinson LL, McGlave PB, Woods WG, Krivit W et al. A randomized study of the prevention of acute graft versus host disease. N Engl J Med 1982; 306: 392–397.
Mohty M, Faucher C, Vey N, Stoppa AM, Viret F, Chabbert I et al. High rate of secondary viral and bacterial infections in patients undergoing allogeneic bone marrow mini-transplantation. Bone Marrow Transplant 2000; 26: 251–255.
Acknowledgements
This work was supported (in part) by the National High Technology Research and Development Program of China (Program 863) (Grant No. 2011AA020105), The Key Program of National Natural Science Foundation of China (Grant No. 81230013), the Scientific Research Foundation for Capital Medicine Development (Grant No. 2011-4022-08) and the National Natural Science Foundation of China (Grant Nos. 30971292 and 30725038). We thank American Journal Experts for reviewing the paper in English. We thank every faculty member who has participated in these studies.
Author contributions
XJH designed and performed the study. YW and HXF collected data. YW and XJH did the data analysis and wrote the paper. All the authors contributed to the interpretation of the data and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Y., Fu, HX., Liu, DH. et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant 49, 426–433 (2014). https://doi.org/10.1038/bmt.2013.191
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.191
Keywords
This article is cited by
-
The lower relapse rate and better survival advantages of haploidentical allograft compared with HLA-matched sibling donor allografts for intermediate- and adverse-risk AML patients with pretransplantation minimal residual disease
Bone Marrow Transplantation (2023)
-
Clinical characteristics and outcomes of Epstein-Barr virus viral load after allogeneic hematopoietic stem cell transplantation
Annals of Hematology (2023)
-
Impact of Anti-T-lymphocyte globulin dosing on GVHD and Immune reconstitution in matched unrelated myeloablative peripheral blood stem cell transplantation
Bone Marrow Transplantation (2022)
-
Risk factors and outcomes of diffuse alveolar haemorrhage after allogeneic haematopoietic stem cell transplantation
Bone Marrow Transplantation (2021)
-
Low-dose anti-thymocyte globulin for GVHD prophylaxis in HLA-matched allogeneic peripheral blood stem cell transplantation
Bone Marrow Transplantation (2021)