Abstract
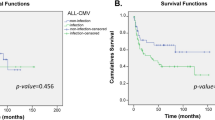
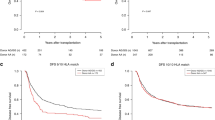
Owing to ethnicity of the population, those best confirmed polymorphisms in the TLR (toll-like receptor)4 and NOD2 genes with significantly prognostic impact on allogeneic hematopoietic SCT (allo-HSCT) seem to be more applicable to Europeans and are nonpolymorphic in the Asian population. The influence of innate immunity gene polymorphisms on the outcomes of allo-HSCT in those populations has been questioned. We evaluated the influence of 10 candidate single nucleotide polymorphisms (SNPs) in the TLR1, TLR2, TLR3, TLR8 and TLR9 genes on the outcomes of allo-HSCT in a Chinese population including 138 pairs of patients and unrelated donors and a second cohort of 102 pairs of patients and HLA-identical sibling donors. We found that two tagSNPs in the TLR9 gene in the donor side, +1174 A/G (rs352139) and +1635 C/T (rs352140), influenced the risk of acute GVHD (aGVHD) and CMV reactivation. Furthermore, the presence of the susceptible haplotype (A–C) in donor may be an informative predicator of worse OS at 5 years compared with those with the G–C and G–T haplotypes (58% vs 82.9%, P=0.024). Our data suggested an unrecognized association between donor TLR9 tagSNPs and the risk of HSCT-related complications in a population without polymorphisms in the TLR4 and NOD2 genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Taylor PA, Ehrhardt MJ, Lees CJ, Panoskaltsis-Mortari A, Krieg AM, Sharpe AH et al. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood 2008; 112: 3508–3516.
Penack O, Holler E, van den Brink MR . Graft-vs-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood 2010; 115: 1865–1872.
Brennan TV, Lin L, Huang X, Cardona DM, Li Z, Dredge K et al. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood 2012; 120: 2899–2908.
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001; 411: 603–606.
Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med 2002; 347: 185–192.
Hugot JP, Zaccaria I, Cavanaugh J, Yang H, Vermeire S, Lappalainen M et al. Prevalence of CARD15/NOD2 mutations in Caucasian healthy people. Am J Gastroenterol 2007; 102: 1259–1267.
Lorenz E, Schwartz DA, Martin PJ, Gooley T, Lin MT, Chien JW et al. Association of TLR4 mutations and the risk for acute GVHD after HLA-matched-sibling hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001; 7: 384–387.
Elmaagacli AH, Koldehoff M, Hindahl H, Steckel NK, Trenschel R, Peceny R et al. Mutations in innate immune system NOD2/CARD 15 and TLR-4 (Thr399Ile) genes influence the risk for severe acute graft-vs-host disease in patients who underwent an allogeneic transplantation. Transplantation 2006; 81: 247–254.
Bochud PY, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med 2008; 359: 1766–1777.
Neumann F, Graef T, Tapprich C, Vaupel M, Steidl U, Germing U et al. Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-vs-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant 2005; 35: 1089–1093.
Ostronoff F, Ostronoff M, Souto-Maior AP, Domingues M, Sucupira A, Manso DA et al. Prospective trial of mycophenolate mofetil-cyclosporine A prophylaxis for acute GVHD after G-CSF stimulated allogeneic bone marrow transplantation with HLA-identical sibling donors in patients with severe aplastic anemia and hematological malignancies. Clin Transplant 2009; 23: 33–38.
Pinana JL, Valcarcel D, Fernandez-Aviles F, Martino R, Rovira M, Barba P et al. MTX or mycophenolate mofetil with CsA as GVHD prophylaxis after reduced-intensity conditioning PBSCT from HLA-identical siblings. Bone Marrow Transplant 2010; 45: 1449–1456.
Huang H, Lin M, Meng H, Qian W, Jin J, Huang J et al. [Combination of mycophenolate mofetil with cyclosporine A and methotrexate as acute GVHD prophylaxis after unrelated donor allogeneic bone marrow transplantation]. Zhonghua Xue Ye Xue Za Zhi 2001; 22: 76–78.
Huang H, Zheng W, Lin M, Fu J, Zhang R . [Prophylaxis of acute graft-vs-host disease after allogeneic bone marrow transplantation by mycophenolate mofetil in a murine model]. Zhonghua Xue Ye Xue Za Zhi 2002; 23: 191–193.
Xiao H, Cao W, Lai X, Luo Y, Shi J, Tan Y et al. Immunosuppressive cytokine gene polymorphisms and outcome after related and unrelated hematopoietic cell transplantation in a chinese population. Biol Blood Marrow Transplant 2011; 17: 542–549.
Xiao HW, Lai XY, Luo Y, Shi JM, Tan YM, He JS et al. Relationship between TNFA, TNFB and TNFRII gene polymorphisms and outcome after unrelated hematopoietic cell transplantation in a Chinese population. Bone Marrow Transplant 2011; 46: 400–407.
Xiao H, Luo Y, Lai X, Fu S, Shi J, Tan Y et al. Genetic variations in T-cell activation and effector pathways modulate alloimmune responses after allogeneic hematopoietic stem cell transplantation in patients with hematologic malignancies. Haematologica 2012; 97: 1804–1812.
Holler E, Rogler G, Brenmoehl J, Hahn J, Herfarth H, Greinix H et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood 2006; 107: 4189–4193.
Mayor NP, Shaw BE, Hughes DA, Maldonado-Torres H, Madrigal JA, Keshav S et al. Single nucleotide polymorphisms in the NOD2/CARD15 gene are associated with an increased risk of relapse and death for patients with acute leukemia after hematopoietic stem-cell transplantation with unrelated donors. J Clin Oncol 2007; 25: 4262–4269.
Nguyen Y, Al-Lehibi A, Gorbe E, Li E, Haagenson M, Wang T et al. Insufficient evidence for association of NOD2/CARD15 or other inflammatory bowel disease-associated markers on GVHD incidence or other adverse outcomes in T-replete, unrelated donor transplantation. Blood 2010; 115: 3625–3631.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097.
Scrucca L, Santucci A, Aversa F . Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 2007; 40: 381–387.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Lazarus R, Klimecki WT, Raby BA, Vercelli D, Palmer LJ, Kwiatkowski DJ et al. Single-nucleotide polymorphisms in the Toll-like receptor 9 gene (TLR9): frequencies, pairwise linkage disequilibrium, and haplotypes in three U.S. ethnic groups and exploratory case-control disease association studies. Genomics 2003; 81: 85–91.
Krieg AM . Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep 2004; 6: 88–95.
Krieg AM . Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene 2008; 27: 161–167.
Calcaterra C, Sfondrini L, Rossini A, Sommariva M, Rumio C, Menard S et al. Critical role of TLR9 in acute graft-vs-host disease. J Immunol 2008; 181: 6132–6139.
Porrett PM, Yuan X, LaRosa DF, Walsh PT, Yang J, Gao W et al. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol 2008; 181: 1692–1699.
Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-vs-host disease. Gut 2010; 59: 1079–1087.
Vaidya SA, Cheng G . Toll-like receptors and innate antiviral responses. Curr Opin Immunol 2003; 15: 402–407.
Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M . Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 2004; 103: 1433–1437.
Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A . Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med 2003; 198: 513–520.
Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA 2004; 101: 3516–3521.
Novak N, Yu CF, Bussmann C, Maintz L, Peng WM, Hart J et al. Putative association of a TLR9 promoter polymorphism with atopic eczema. Allergy 2007; 62: 766–772.
Tao K, Fujii M, Tsukumo S, Maekawa Y, Kishihara K, Kimoto Y et al. Genetic variations of Toll-like receptor 9 predispose to systemic lupus erythematosus in Japanese population. Ann Rheum Dis 2007; 66: 905–909.
Bochud PY, Hersberger M, Taffe P, Bochud M, Stein CM, Rodrigues SD et al. Polymorphisms in Toll-like receptor 9 influence the clinical course of HIV-1 infection. AIDS 2007; 21: 441–446.
Elmaagacli AH, Koldehoff M, Beelen DW . Improved outcome of hematopoietic SCT in patients with homozygous gene variant of Toll-like receptor 9. Bone Marrow Transplant 2009; 44: 295–302.
Elmaagacli AH, Steckel N, Ditschkowski M, Hegerfeldt Y, Ottinger H, Trenschel R et al. Toll-like receptor 9, NOD2 and IL23R gene polymorphisms influenced outcome in AML patients transplanted from HLA-identical sibling donors. Bone Marrow Transplant 2011; 46: 702–708.
Bafica A, Scanga CA, Schito M, Chaussabel D, Sher A . Influence of coinfecting pathogens on HIV expression: evidence for a role of Toll-like receptors. J Immunol 2004; 172: 7229–7234.
Schlaepfer E, Audige A, von Beust B, Manolova V, Weber M, Joller H et al. CpG oligodeoxynucleotides block human immunodeficiency virus type 1 replication in human lymphoid tissue infected ex vivo. J Virol 2004; 78: 12344–12354.
Schlaepfer E, Audige A, Joller H, Speck RF . TLR7/8 triggering exerts opposing effects in acute vs latent HIV infection. J Immunol 2006; 176: 2888–2895.
Medzhitov R, Janeway C Jr. . The Toll receptor family and microbial recognition. Trends Microbiol 2000; 8: 452–456.
Chiffoleau E, Heslan JM, Heslan M, Louvet C, Condamine T, Cuturi MC . TLR9 ligand enhances proliferation of rat CD4+ T cell and modulates suppressive activity mediated by CD4+ CD25+ T cell. Int Immunol 2007; 19: 193–201.
Li JL, Song WR, Czerwinski DK, Varghese B, Uematsu S, Akira S et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol 2007; 179: 2493–2500.
Houot R, Levy R . T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood 2009; 113: 3546–3552.
Acknowledgements
We would like to thank the Shanghai Genesky Bio-Tech Genetic Core Lab for their excellent technical assistance with sequencing analysis. We also thank the HLA Typing Laboratory of the Blood Center of the Zhejiang Province for their assistance. This work was supported in part by the Key Project of National Natural Science Foundation of China (81230014), National High Technology Research and Development Program of China (2012AA020905), National Natural Science Foundation of China (81100387, 81170501), foundation of the Zhejiang Educational Committee (Y201019151) and foundation of the Zhejiang Science Technology Department (N20080398).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Xiao, H., Luo, Y., Lai, X. et al. Donor TLR9 gene tagSNPs influence susceptibility to aGVHD and CMV reactivation in the allo-HSCT setting without polymorphisms in the TLR4 and NOD2 genes. Bone Marrow Transplant 49, 241–247 (2014). https://doi.org/10.1038/bmt.2013.160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.160
Keywords
This article is cited by
-
Sensing danger: toll-like receptors and outcome in allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2017)
-
The Microbiome and Allogeneic Stem Cell Transplantation
Current Stem Cell Reports (2015)