Abstract
The human recombinant G-CSF filgrastim has been widely used for the mobilization of CD34+ stem cells of healthy donors (HD). In 2008, the G-CSF biosimilar XM02 (Ratiograstim, Tevagrastim and Biograstim) was approved by the European Medicines Agency (EMA) for the mobilization of PBSC. However, there is limited experience in the application of biosimilar G-CSF for the mobilization of PBSC especially in HD. Therefore, we investigated in two cohorts (n=22), the efficacy and safety of PBSC mobilization by either biosimilar G-CSF or reference G-CSF. We observed a similar yield of CD34+ stem cells as well as CD3+ T-cells and nucleated cells in both groups and a safe engraftment in all patients with similar reconstitution of hematopoiesis in all hosts. In summary, we found a comparable efficacy and safety of biosimilar G-CSF when compared with reference G-CSF.
Similar content being viewed by others
Introduction
G-CSF has become a very powerful and widely used tool in the hands of hematologists and oncologists to treat neutropenia caused either by disease or therapy, such as chemotherapy, irradiation or low engraftment after hematopoietic SCT.1, 2 When conventional drugs produced by chemical synthesis contain the same active substance like the original drug but different formulation, this is called generics. For generic drugs in the field of growth factors like G-CSF that are manufactured by the use of recombinant technology, the term ‘biosimilars’ has been coined.3 There is an ongoing debate on the efficacy and safety of biosimilars.4, 5, 6, 7
The biosimilar G-CSF XM02 (Ratiograstim, Tevagrastim and Biograstim) was fully approved in 2008 by the European Medicines Agency (EMA) for all indications of the reference filgrastim (Neupogen), that is, use for patients with chemotherapy-induced neutropenia, for the mobilization of stem cells in the autologous and allogeneic settings, for patients with agranulocytosis, and for patients with neutropenia due to infection with HIV.8 Comparability of the biosimilar G-CSF with the reference was assessed in a comparative study with regard to a single indication for which the reference G-CSF is approved, that is, the efficacy of the G-CSFs against chemotherapy-induced neutropenia patients with breast cancer. Further studies compared the effect of the G-CSFs in patients with lung cancer and non-Hodgkin’s lymphoma.9 The extrapolation from these positive data to the use of the biosimilar G-CSF for the mobilization of CD34+ stem cells in healthy donors (HD) was based on European Law.8 However, extrapolation in general raised questions.3, 4, 5, 7, 10
The European Group for Blood and Bone Marrow Transplantation (EBMT) announced concerns of the use of biosimilar G-CSF in HD until efficacy and safety data have been collected in clinical trials in the autologous setting, encompassing an adequate number of stem cell mobilization procedures with adequate follow-up.11
Therefore, we initiated here a prospective study in HD and patients with hematological malignancies undergoing allo-SCT.
Materials and methods
Twenty-two patients undergoing allo-SCT for hematological malignancies and their related HD were investigated in a pilot study at a single institution, the Stem Cell Transplantation Unit of the University Clinic of Rostock, Rostock, Germany. The federal authority, that is, the ‘Bundesamt fuer Pharmazeutische Arzneimittel (BfArM)’, was informed about the pilot study which was registered (ISRCTN94372129).
Eleven donors at a median age of 58 years (s.d.±12 years, range: 28–72 years) received biosimilar G-CSF, and eleven donors at a median age of 51 years (s.d.±11 years, range: 27–68 years) received control G-CSF. Donors received a standard dose of 10 μg/kg BW biosimilar vs reference G-CSF s.c. BID for four days. On the morning of the 5th day, the ninth dose was applied and 2 hours later, the HD was put on leukapheresis. The target leukapheresis yield was 5 × 106 CD34+ cells/kg BW of the recipient. If a second round of leukapheresis was necessary, the donor received a 10th dose on the evening of the 5th day, and an 11th dose on the morning of the 6th day.
The patients for allo-SCT suffered from acute and chronic leukemia or Non-Hodgkin’s lymphoma. One cohort of donors received control G-CSF, and the second cohort was administered biosimilar G-CSF. During the whole period, the same standard operating procedures for the mobilization and collection of stem cells from donors as well as the SOPs for the treatment of patients with allo-SCT were followed. G-CSF was only used for mobilization, but no G-CSF was directly applied to the patients. In either group, 10/11 patients received a non-myeloablative conditioning regimen.
The data were evaluated for the following parameters: (1) WBC count in the peripheral blood after mobilization, (2) CD34+ cell count after mobilization, (3) CD34+ cell count absolute numbers and CD34+ cells per kg body weight of the patients, (4) number of leukapheresis procedures, (5), number of CD3+ T lymphocytes, (6) number of nucleated cells in the graft, (7) time till regeneration of WBC, neutrophils and platelets and (8) side effects.
The values for the parameters obtained with the G-CSF biosimilar vs the control group were tested for Gaussian distribution using the D'Agostino and Pearson omnibus normality test. For all parameters of the donors, this test assumed Gaussian distribution and the unpaired t-test was used for significance testing. For the parameters characterizing the patients’ reconstitution, Gaussian distribution could not be assumed according to D’Agostino and Pearson’s test, and therefore significance was evaluated by the Mann–Whitney test.
Results
Different parameters to characterize the yield and the composition of the graft are displayed in Figure 1 as dot plots. In HDs receiving the G-CSF biosimilar XM02 subcutaneously, WBC counts in the peripheral blood ranged from 29.9 G/L to 64.6 G/L (median 50.8 G/L) and the CD34+ cell counts from 19.3/mm3 to 114.6/mm3 (median 65.8/mm3). In a mean of 1.45 leukapheresis procedures, 173 × 106–494 × 106 CD34+ cells (median 356 × 106) were collected. This resulted in CD34+ cell numbers of 2.0 × 106–7.3 × 106 (median 4.4 × 106) per kg body weight of the patients. Grafts contained 0.7–2.6 × 1010 (median 1.2 × 1010) CD3+ T-cells and 3.7–9.9 × 1010 (median 7.2 × 1010) nucleated cells. In HDs receiving the reference G-CSF subcutaneously, WBC counts ranged from 27.1 G/L to 62.5 G/L (median 43.3 G/L) and the CD34+ cell counts from 13.6/mm3 to 122.4/mm3 (median 50.9/mm3). A mean of 1.27 leukapheresis procedures were necessary to collect 182 × 106–598 × 106 CD34+ cells (median 358 × 106) absolutely. This translates into 2.1 × 106 7.9 × 106 (median 4.2 × 106) CD34+ cells per kg body weight of the patients. Grafts contained 1.0–2.3 × 1010 (median 1.5 × 1010) CD3+ T-cells and 3.6–9.6 × 1010 (median 5.1 × 1010) nucleated cells.
Comparison of PBSC mobilized by either the biosimilar XM02 (Ratiograstim, Tevagrastim and Biograstim) (upper panel) or the reference G-CSF (lower panel). The first two dot plots on the left display the WBC and CD34+ stem cell counts in the matched related donors. The two dot plots in the middle give the content of CD34+ stem cells in the leukapheresis product, the two dot plots on the right the absolute count of CD3+ T cells and nucleated cells in the product.
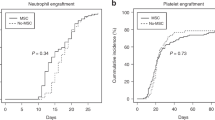
Figure 2 summarizes the engraftment of stem cells mobilized by either reference G-CSF or the biosimilar XM02. In the biosimilar XM02 cohort, patients’ WBC regenerated >0.5 G/L and >1 G/L within a median of 12 days (range 10–19 days) and 13 days (range 11–20 days) respectively. Neutrophils regenerated >0.5 G/L within a median of 14 days (range 11–20 days). Platelets reached counts >20 G/L within a median of 6 days (range 0–8 days), and >50 G/L within a median of 13 days (range 11–46 days). In the reference G-CSF group, patients regenerated leukocytes >0.5 G/L within a median of 14 days (range 9–18 days) and >1 G/L within a median of 16 days (range 10–30 days). Neutrophils achieved values >0.5 G/L within a median of 17 days (range 11–34 days). Platelets were >20 G/L within a median of 8 days (range 0–16 days), and >50 G/L within a median of 16 days (range 10–25 days).
Hematopoietic reconstitution of the host. Recovery of WBC, neutrophils and platelets was assessed for the thresholds as indicated. The patients received a graft from their matched related donors after mobilization with the biosimilar XM02 (Ratiograstim, Tevagrastim and Biograstim) (upper panel) or the reference G-CSF (lower panel).
In total, we analyzed 22 patients and 22 donors, one cohort receiving the biosimilar XM02 versus another cohort receiving reference G-CSF. As for side effects, neither allergic reactions nor alterations in kidney and liver function were observed in the donors. In both the groups, 6/11 donors reported arthralgias during the time of stimulation with G-CSF.
The lack of significant differences for these parameters for grafts obtained after mobilization with the biosimilar XM02 (Ratiograstim, Tevagrastim and Biograstim) versus reference G-CSF clearly demonstrate the ‘similarity’ of the biosimilar in terms of efficacy and safety.
Discussion
In comparison to the reference group, we did not see significant differences in (1) WBC count in the peripheral blood after mobilization, (2) CD34+ cell count after mobilization, (3) CD34+ cell count absolute numbers and CD34+ cells per kg body weight of the patients, (4) number of leukapheresis procedures, (5) number of CD3+ T lymphocytes, (6). number of nucleated cells in the graft and (7) regeneration of WBC, neutrophils and platelets. All patients engrafted, and (8) only expected side effects like arthalgias were observed.
Biosimilars have not been available on the market as long as the original. Therefore long-term safety for both the donor and recipient of the graft can be only evaluated over the forthcoming years. Nevertheless, neither graft rejection nor side effects occurred more frequently than we expected from the reference G-CSF. Moreover, pharmacovigilance data from the company producing XM02 are now based on more than 150 000 patients (data on file) who received XM02 because of neutropenia after chemotherapy for a solid tumor or leukemia and lymphoma.12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Neither serious side effects exceeding CTC grade 2 toxicity nor secondary malignoma after the use of XM02 have been recorded. The plethora of these data in patients with neutropenia after chemotherapy for cancer in conjunction with our results in a cohort of recipients of allogeneic stem cells from a HLA-matched related donor (MRD) shows that mobilization with the G-CSF biosimilar XM02 is feasible and safe.
In this pilot study, the efficacy of the G-CSF biosimilar XM02 (Ratiograstim, Tevagrastim and Biograstim) was similar for yield and engraftment as well as for the frequency of side effects in the context of allo-SCT when compared with the reference G-CSF. Our results do not support concerns about the use of the G-CSF biosimilar XM02 for the mobilization of stem cells in HDs.
References
Aapro MS, Cameron DA, Pettengell R, Bohlius J, Crawford J, Ellis M et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumors. Eur J Cancer 2006; 42: 2433–2453.
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L et al. American Society of Clinical Oncology. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based, clinical practice guideline. J Clin Oncol 2006; 24: 3187–3205.
Dingermann T . Recombinant therapeutic proteins: production platforms and challenges. Biotechnol J 2008; 3: 90–97.
Niederwieser D, Schmitz S . Biosimilar agents in oncology/haematology: from approval to practice. Eur J Haematol 2011; 86: 277–288.
Gascon P . Presently available biosimilars in hematology-oncology: G-CSF. Target Oncol 2012; 7 (Suppl 1): 29–34.
Schofield I . EC defends biosimilar safety. Scrip 2008; 1: 3358.
Mellstedt H, Niederwieser D, Ludwig H . The challenge of biosimilars. Ann Oncol 2008; 19: 411–419.
European Medicines Agency Assessment Report for Ratiograstim. Nonproprietary Name: filgrastim (EMEA/H/C/825) Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000825/WC500047793.pdf.
Engert A, del Giglio A, Bias P, Lubenau H, Gatzemeier U, Heigener D . Incidence of febrile neutropenia and myelotoxicity of chemotherapy: a meta-analysis of biosimilar G-CSF studies in breast cancer, lung cancer, and non-Hodgkin’s lymphoma. Onkologie 2009; 32: 599–604.
Sheridan B, Fox A . Feature on biopharmaceuticals: views from the pharmaceutical industry - significant questions relating to efficacy and immunogenicity will remain at approval. Eur J Hosp Pharmacy Practice 2007; 13: 70–73.
European Group for Blood and Marrow Transplantation (EBMT) Position statement: biosimilar granulocyte-colony stimulating factor (G-CSF) for stem cell mobilization in related and unrelated donors Available from http://www.worldmarrow.org/fileadmin/Committees/CLWG/Biosimilars/Biosimilars_9Jan09.pdf.
del Giglio A, Eniu A, Ganae-Motan D, Topuzov E, Lubenau H . XM02 is superior to placebo and equivalent to NeupogenTM in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle I in breast cancer patients receiving decetaxel/doxorubicin chemotherapy. BCM Cancer 2008; 8: 332–339.
Lubenau H, Sveikata A, Gumbrevicius G, Macijauskiene J, Fokas V, Kazlauskas S et al. Bioequivalence of two recombinant granulocyte colony-stimulating factor products after subcutaneous injection in healthy volunteers. Int J Clin Pharm Therap 2009; 47: 275–282.
Lubenau H, Bias P, Maly A-K, Siegler KE, Mehltretter K . Pharmakokinetic and pharmacodynamic profile of new biosimilar Filgrastim XM02 equivant to market Filgrastim Neupogen. Biodrugs 2009; 23: 43–51.
Gatzemeier U, Ciuleanu T, Dediu M, Ganea-Motan E, Lubenau H, del Giglio A . XM02, the first biosimilar G-CSF, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with small cell or non-small cell lung cancer receiving platinum-based chemotherapy. J Thorac Oncol 2009; 4: 736–740.
Engert A, Griskevicius L, Zyuzgin Y, Lubenau H, del Giglio A . XM02, the first granulocyte conoly-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk Lymphoma 2009; 50: 374–379.
Schmitt M, Diestel L, Xu X, Borchert K, Gläser D, Hilgendorf I et al. Application of the G-CSF biosimilar Ratiograstim® for the mobilisation of peripheral stem cells in healthy donors. Onkologie 2011; 34 (Suppl 6): P926.
Andreola G, Babic A, Rabascio C, Negri M, Martinelli G, Laszlo D . Plerixafor and Filgrastim XM02 (Tevagastrim) as a first line peripheral blood stem cell mobilisation strategy in patients with multiple myeloma and lymphoma candidated to autologous bone marrow transplantation. Eur J Haematol 2011; 88: 154–158.
Laszlo D, Andreola G, Babic A, Rabascio C, Montinaro A, Morabito L et al. Martinelli Plerixafor in combination with originator or biosimilar XM02-G-CSF as first-line peripheral blood stem cell mobilisation strategy in patients with lymphomas and multiple myeloma candidate to ASCT: a single-centre experience. Bone Marrow Transplant 2012; 47: P709.
Sever M, Domanovic D, Vrtovec B, Lezaic L, Pretnar J, Poglajen G et al. Mobilisation of heart failure patients using biosimilar granulocyte colony stimulating factor (TevaGrastim) for autologous CD34+ cells preparation and application into the heart. Bone Marrow Transplant 2012; 47: P726.
Publicover A, Richardson DS, Hill K, Hurlock C, Casey P, Newman J et al. Use of biosimilar G-CSF for peripheral blood progenitor cell mobilisation prior to autologous stem cell transplantation: a single-centre experience. Bone Marrow Transplant 2010; 45: P595.
Acknowledgements
This work was supported by a grant from the Ratiopharm Direct Ltd., Ulm, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MS and MF received travel grants and speakers’ honoraria from both Amgen Ltd., Germany and Teva/Ratiopharm Direct Ltd., Germany. IH received travel grant from Ratiopharm Direct Ltd., Germany.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Schmitt, M., Xu, X., Hilgendorf, I. et al. Mobilization of PBSC for allogeneic transplantation by the use of the G-CSF biosimilar XM02 in healthy donors. Bone Marrow Transplant 48, 922–925 (2013). https://doi.org/10.1038/bmt.2012.270
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.270
Keywords
This article is cited by
-
The safety and efficacy of hematopoietic stem cell mobilization using biosimilar filgrastim in related donors
International Journal of Hematology (2022)
-
Current use of biosimilar G-CSF for haematopoietic stem cell mobilisation
Bone Marrow Transplantation (2019)
-
Tbo-Filgrastim: A Review in Neutropenic Conditions
BioDrugs (2016)