Abstract
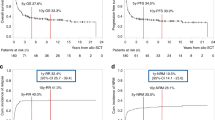
Childhood autologous hematopoietic cell transplant (auto-HCT) survivors can be at risk for secondary malignant neoplasms (SMNs). We assembled a cohort of 1487 pediatric auto-HCT recipients to investigate the incidence and risk factors for SMNs. Primary diagnoses included neuroblastoma (39%), lymphoma (26%), sarcoma (18%), central nervous system tumors (14%) and Wilms tumor (2%). Median follow-up was 8 years (range, <1–21 years). SMNs were reported in 35 patients (AML/myelodysplastic syndrome (MDS)=13, solid cancers=20, subtype missing=2). The overall cumulative incidence of SMNs at 10 years from auto-HCT was 2.60% (AML/MDS=1.06%, solid tumors=1.30%). We found no association between SMNs risk and age, gender, diagnosis, disease status, time since diagnosis or use of TBI or etoposide as part of conditioning. OS at 5-years from diagnosis of SMNs was 33% (95% confidence interval (CI), 16–52%). When compared with age- and gender-matched general population, auto-HCT recipients had 24 times higher risks of developing SMNs (95% CI, 16.0–33.0). Notable SMN sites included bone (N=5 SMNs, observed (O)/expected (E)=81), thyroid (N=5, O/E=53), breast (N=2, O/E=93), soft tissue (N=2, O/E=34), AML (N=6, O/E=266) and MDS (N=7, O/E=6603). Risks of SMNs increased with longer follow-up from auto-HCT. Pediatric auto-HCT recipients are at considerably increased risk for SMNs and need life-long surveillance for SMNs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Matthay KK . Intensification of therapy using hematopoietic stem-cell support for high-risk neuroblastoma. Pediatr Transplant 1999; 3 (Suppl 1): 72–77.
Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M et al. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol 2002; 20: 2284–2292.
Stram DO, Matthay KK, O’Leary M, Reynolds CP, Haase GM, Atkinson JB et al. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: a report of two concurrent Children’s Cancer Group studies. J Clin Oncol 1996; 14: 2417–2426.
Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 2006; 7: 813–820.
Lieskovsky YE, Donaldson SS, Torres MA, Wong RM, Amylon MD, Link MP et al. High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric Hodgkin’s disease: results and prognostic indices. J Clin Oncol 2004; 22: 4532–4540.
Burke MJ, Walterhouse DO, Jacobsohn DA, Duerst RE, Kletzel M . Tandem high-dose chemotherapy with autologous peripheral hematopoietic progenitor cell rescue as consolidation therapy for patients with high-risk Ewing family tumors. Pediatr Blood Cancer 2007; 49: 196–198.
Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2010; 102: 1083–1095.
Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol 2009; 27: 2356–2362.
Meadows AT, Baum E, Fossati-Bellani F, Green D, Jenkin RD, Marsden B et al. Second malignant neoplasms in children: an update from the Late Effects Study Group. J Clin Oncol 1985; 3: 532–538.
Hijiya N, Hudson MM, Lensing S, Zacher M, Onciu M, Behm FG et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 2007; 297: 1207–1215.
Walter AW, Hancock ML, Pui CH, Hudson MM, Ochs JS, Rivera GK et al. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J Clin Oncol 1998; 16: 3761–3767.
Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood 2005; 105: 3802–3811.
Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB et al. Solid cancers after bone marrow transplantation. N Engl J Med 1997; 336: 897–904.
Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood 2011; 117: 316–322.
Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood 2009; 113: 1175–1183.
Clark TG, Altman DG, De Stavola BL . Quantification of the completeness of follow-up. Lancet 2002; 359: 1309–1310.
Klein J, Moeschberger M . Survival analysis: techniques of censored and truncated Data. 2nd edn. Springer-Verlag: New York, 2003.
Kaplan EL, Meier P . Nonparametric estimation from in complete observations. J Am Stat Assoc 1958; 53: 457–481.
Cox DR . Regression models and life tables. J R Stat Soc 1972; 34: 187–202.
Breslow NE, Day NE . The design and analysis of cohort studies. In: Heseltine E (ed.),. Statistical Methods in Cancer Research. Vol II. International Agency for Research on Cancer Scientific Publications No. 82: Lyon, France, 1987.
Preston DL, Lubin JH, Pierce DA . Epicure User’s Guide. Hirosoft International: Seattle, WA, 1993.
Curado MP, Edwards B, Shin HR et al. Cancer Incidence in Five Continents. Vol IX. International Agency for Research on Cancer Scientific Publications No. 160. Lyon, France, 2007.
Horner MJ, Ries LAG, Krapcho M et al. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute, Bethesda, MD. In: http://seer.cancer.gov/1975_2006/ based on November 2008 SEER data submission, posted to the SEER web site 2009.
Nottage K, Lanctot J, Li Z, Neglia JP, Bhatia S, Hammond S et al. Long-term risk for subsequent leukemia after treatment for childhood cancer: a report from the Childhood Cancer Survivor Study. Blood 117: 6315–6318.
Hake CR, Graubert TA, Fenske TS . Does autologous transplantation directly increase the risk of secondary leukemia in lymphoma patients? Bone Marrow Transplant 2007; 39: 59–70.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; the Leukemia and Lymphoma Society; the Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense or any other agency of the US Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Danner-Koptik, K., Majhail, N., Brazauskas, R. et al. Second malignancies after autologous hematopoietic cell transplantation in children. Bone Marrow Transplant 48, 363–368 (2013). https://doi.org/10.1038/bmt.2012.166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.166
Keywords
This article is cited by
-
Characteristics and outcomes of children, adolescents and young adults with relapsed/refractory non-hodgkin lymphoma undergoing autologous stem cell transplant
BMC Cancer (2023)
-
Male-specific late effects in adult hematopoietic cell transplantation recipients: a systematic review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and Transplant Complications Working Party of the European Society of Blood and Marrow Transplantation
Bone Marrow Transplantation (2022)
-
Risk of developing second malignant neoplasms in patients with neuroblastoma: a population study of the US SEER database
Radiation Oncology (2021)
-
Secondary cancer after a childhood cancer diagnosis: viewpoints considering primary cancer
International Journal of Clinical Oncology (2018)
-
Subsequent malignant neoplasms in pediatric cancer patients treated with and without hematopoietic SCT
Bone Marrow Transplantation (2015)