Abstract
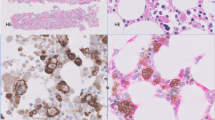
Dapsone (4-4′-diaminodiphenylsulfone) is commonly used for Pneumocystis jirovecii pneumonia (PCP) prophylaxis in immunocompromised patients. Oxidant hemolysis is a known complication of dapsone, but its frequency in adult patients who have undergone a SCT for hematological malignancies is not well established. We studied the presence of oxidant hemolysis, by combining examination of RBC morphology and laboratory data, in 30 patients who underwent a SCT and received dapsone for PCP prophylaxis, and compared this group with 26 patients who underwent a SCT and received trimethoprim-sulfamethoxazole (TMP-SMX) for PCP prophylaxis. All patients had normal glucose-6-phosphate dehydrogenase (G6PDH) enzymatic activity. In SCT patients, dapsone compared with TMP-SMX for PCP prophylaxis was associated with a high incidence of oxidant hemolysis (87 vs 0%, P<0.001), and the morphological evaluation of oxidant hemolysis correlated well with laboratory evidence of hemolysis. Dapsone-induced oxidant hemolysis in SCT patients is 20-fold higher than the reported rate in the population of HIV-infected patients, and thus much higher than the prevalence of G6PDH variants in the general population. In our patients, it manifested clinically as a lower Hb that was not significant enough to result in increased packed RBC transfusions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Boeckh M, Marr KA . Infection in hematopoietic stem cell transplantation. In: Rubin RH, Young LS (eds). Clinical Approach to Infection in the Compromised Host. Kluwer Academic/Plenum: New York, 2002, pp 527–571.
Castro M . Treatment and prophylaxis of Pneumocystis carinii pneumonia. Semin Respir Infect 1998; 13: 296–303.
Hughes WT . Use of dapsone in the prevention and treatment of Pneumocystis carinii pneumonia: a review. Clin Infect Dis 1998; 27: 191–204.
Marras TK, Sanders K, Lipton JH, Messner HA, Conly J, Chan CK . Aerosolized pentamidine prophylaxis for Pneumocystis carinii pneumonia after allogeneic marrow transplantation. Transpl Infect Dis 2002; 4: 66–74.
El-Sadr WM, Murphy RL, Yurik TM, Luskin-Hawk R, Cheung TW, Balfour Jr HH et al. Atovaquone compared with dapsone for the prevention of Pneumocystis carinii pneumonia in patients with HIV infection who cannot tolerate trimethoprim, sulfonamides, or both. Community Program for Clinical Research on AIDS and the AIDS Clinical Trials Group. N Engl J Med 1998; 339: 1889–1895.
Zhu YI, Stiller MJ . Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol 2001; 45: 420–434.
Bluhm RE, Adedoyin A, McCarver DG, Branch RA . Development of dapsone toxicity in patients with inflammatory dermatoses: activity of acetylation and hydroxylation of dapsone as risk factors. Clin Pharmacol Ther 1999; 65: 598–605.
Beumont MG, Graziani A, Ubel PA, MacGregor RR . Safety of dapsone as Pneumocystis carinii pneumonia prophylaxis in human immunodeficiency virus-infected patients with allergy to trimethoprim/sulfamethoxazole. Am J Med 1996; 100: 611–616.
Grossman SJ, Jollow DJ . Role of dapsone hydroxylamine in dapsone-induced hemolytic anemia. J Pharmacol Exp Ther 1988; 244: 118–125.
Jollow DJ, Bradshaw TP, McMillan DC . Dapsone-induced hemolytic anemia. Drug Metab Rev 1995; 27: 107–124.
Centers for Disease Control and Prevention; Infectious Disease Society of America; American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep 2000; 49 (RR-10): 1–125, CE121-127.
Perkins S . Glucose-6-Phosphate dehygrogenase deficiency. In: Kjelsdsberg CR (ed) Practical Diagnosis of Hematologic Disorders, ol. 1. ASCP Press: Chicago, 2006, pp 113–122.
Naik PM, Lyon III GM, Ramirez A, Lawrence EC, Neujahr DC, Force S et al. Dapsone-induced hemolytic anemia in lung allograft recipients. J Heart Lung Transplant 2008; 27: 1198–1202.
Lee I, Barton TD, Goral S, Doyle AM, Bloom RD, Chojnowski D et al. Complications related to dapsone use for Pneumocystis jirovecii pneumonia prophylaxis in solid organ transplant recipients. Am J Transplant 2005; 5: 2791–2795.
Sangiolo D, Storer B, Nash R, Corey L, Davis C, Flowers M et al. Toxicity and efficacy of daily dapsone as Pneumocystis jiroveci prophylaxis after hematopoietic stem cell transplantation: a case-control study. Biol Blood Marrow Transplant 2005; 11: 521–529.
Rodriguez M, Fishman JA . Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev 2004; 17: 770–782.
Masur H, Kaplan JE, Holmes KK . Guidelines for preventing opportunistic infections among HIV-infected persons--2002. Recommendations of the US. Public health service and the infectious diseases society of America. Ann Intern Med 2002; 137 (Part 2): 435–478.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Olteanu, H., Harrington, A., George, B. et al. High prevalence of Dapsone-induced oxidant hemolysis in North American SCT recipients without glucose-6-phosphate-dehydrogenase deficiency. Bone Marrow Transplant 47, 399–403 (2012). https://doi.org/10.1038/bmt.2011.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.83
Keywords
This article is cited by
-
Dapsone induced hemolysis in a patient with ANCA associated glomerulonephritis and normal G6PD level and implications for clinical practice: case report and review of the literature
SpringerPlus (2015)
-
Pneumocystis Pneumonia: Epidemiology and Options for Prophylaxis in Non-HIV Immunocompromised Pediatric Patients
Current Fungal Infection Reports (2014)
-
Hypersensitivity reactions to non beta-lactam antimicrobial agents, a statement of the WAO special committee on drug allergy
World Allergy Organization Journal (2013)