Abstract
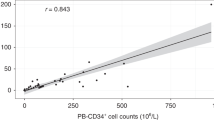
Plerixafor is an inhibitor of CXCR-4 (CXC chemokine receptor-4)/SDF (stromal cell-derived factor)-1 binding used in combination with granulocyte colony-stimulating factor (G-CSF) for mobilization of autologous peripheral blood hematopoietic stem cells (HSCs). We developed a data-generated, cost-saving decision-making algorithm that uses the CD34+ count in the peripheral blood on the fourth day of G-CSF administration (PB-CD34+), and the collection target (T-CD34+) to decide between continuing G-CSF only (G approach) or adding plerixafor to the mobilization regimen (G+P approach) aiming at the lowest cost. The G+P approach was more cost-effective with lower PB-CD34+. It was possible to determine, for each T-CD34+, the maximum PB-CD34+ for which the G+P approach is cost-effective, generating an algorithm for the use of plerixafor. We validated this algorithm in a cohort of 34 patients undergoing HSC mobilization. In all, 11 patients completed collection on the G approach and 23 patients on the G+P approach, with 91% of the patients completing collection within the predicted number of apheresis sessions. All patients who underwent transplantation engrafted with minimal differences in engraftment time between G and G+P approaches. This validated algorithm provides a potential cost-saving decision tool for the use of plerixafor in autologous HSC mobilization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50–70: results of a randomized controlled trial. Blood 2004; 104: 3052–3057.
Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet 2002; 359: 2065–2071.
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–1545.
Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson HE et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol 2003; 21: 3918–3927.
Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet 1996; 347: 353–357.
Vose JM, Sharp G, Chan WC, Nichols C, Loh K, Inwards D et al. Autologous transplantation for aggressive non-Hodgkin's lymphoma: results of a randomized trial evaluating graft source and minimal residual disease. J Clin Oncol 2002; 20: 2344–2352.
Gertz MA, Kumar SK, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK et al. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant 2009; 43: 619–625.
Moskowitz CH, Glassman JR, Wuest D, Maslak P, Reich L, Gucciardo A et al. Factors affecting mobilization of peripheral blood progenitor cells in patients with lymphoma. Clin Cancer Res 1998; 4: 311–316.
Micallef IN, Apostolidis J, Rohatiner AZ, Wiggins C, Crawley CR, Foran JM et al. Factors which predict unsuccessful mobilisation of peripheral blood progenitor cells following G-CSF alone in patients with non-Hodgkin's lymphoma. Hematol J 2000; 1: 367–373.
Alegre A, Tomas JF, Martinez-Chamorro C, Gil-Fernandez JJ, Fernandez-Villalta MJ, Arranz R et al. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant 1997; 20: 211–217.
Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol 1998; 16: 1547–1553.
Kuittinen T, Nousiainen T, Halonen P, Mahlamaki E, Jantunen E . Prediction of mobilisation failure in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant 2004; 33: 907–912.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia 2007; 21: 2035–2042.
Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant 2009; 15: 718–723.
Stewart DA, Smith C, MacFarland R, Calandra G . Pharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myeloma. Biol Blood Marrow Transplant 2009; 15: 39–46.
Lack NA, Green B, Dale DC, Calandra GB, Lee H, MacFarland RT et al. A pharmacokinetic-pharmacodynamic model for the mobilization of CD34+ hematopoietic progenitor cells by AMD3100. Clin Pharmacol Ther 2005; 77: 427–436.
Tricot G, Cottler-Fox MH, Calandra G . Safety and efficacy assessment of plerixafor in patients with multiple myeloma proven or predicted to be poor mobilizers, including assessment of tumor cell mobilization. Bone Marrow Transplant 2009; 45: 63–68.
Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M et al. Treatment with plerixafor in non-Hodgkin's lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biol Blood Marrow Transplant 2009; 15: 249–256.
DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009; 113: 5720–5726.
DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol 2009; 27: 4767–4773.
Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 2005; 106: 1867–1874.
Vose JM, Ho AD, Coiffier B, Corradini P, Khouri I, Sureda A et al. Advances in mobilization for the optimization of autologous stem cell transplantation. Leuk Lymphoma 2009; 50: 1412–1421.
Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia 2009; 23: 1904–1912.
Micallef I, Ansell SM, Buadi FK, Dingli D, Dispenzieri A, Gastineau DA et al. A risk adapted approach utilizing plerixafor in autologous peripheral blood stem cell mobilization. Blood 2009; 114: 3211 (abstract).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Costa, L., Alexander, E., Hogan, K. et al. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplant 46, 64–69 (2011). https://doi.org/10.1038/bmt.2010.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.78
Keywords
This article is cited by
-
On demand plerixafor is safe and effective for hematopoietic progenitor cell mobilization in patients with light chain amyloidosis at risk for mobilization failure with G-CSF alone
Bone Marrow Transplantation (2023)
-
Development and validation of a predictive model to guide the use of plerixafor in pediatric population
Bone Marrow Transplantation (2022)
-
Does use of biosimilar G-CSF change plerixafor utilization during stem cell mobilization for autologous stem cell transplant?
Bone Marrow Transplantation (2020)
-
Plerixafor in non-Hodgkin’s lymphoma patients: a German analysis of time, effort and costs
Bone Marrow Transplantation (2019)
-
Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement
Bone Marrow Transplantation (2019)