Abstract
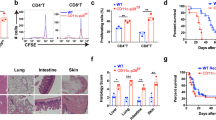
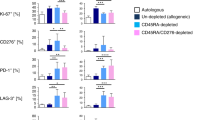
Previously, we have shown that IL-21R−/− splenocytes ameliorate GVHD as compared with wild-type splenocytes. Here, we investigated whether or not IL-21R−/− splenocytes diminish the graft-versus-leukemia (GVL) effect. Surprisingly, IL-21R−/− splenocytes efficiently eliminate leukemic cells as well as wild-type splenocytes, suggesting the retention of GVL effects in the absence of IL-21 signaling. To compare the GVL effect between IL-21R−/− and wild-type cells, we titrated the number of splenocytes required for the elimination of leukemic cells and found that the threshold of GVL effect was obtained between 5 × 105 and 5 × 106 with both types of splenocytes. Cotransplantation with CD8-depleted splenocytes but not with purified CD8 T-cells resulted in a significant reduction in anti-leukemic effect of IL-21R−/− cells compared with wild-type cells, suggesting that the lack of IL-21 signaling primarily impairs CD4 T-cell rather than CD8 T-cell function and the comparable GVL effect with IL-21R−/− bulk splenocytes results from cooperative compensation by CD8 T-cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ozaki K, Leonard WJ . Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem 2002; 277: 29355–29358.
Leonard WJ, Spolski R . Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol 2005; 5: 688–698.
Leonard WJ, Zeng R, Spolski R . Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol 2008; 84: 348–356.
Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408: 57–63.
Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ . Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA 2000; 97: 11439–11444.
Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A et al. A critical role for IL-21 in regulating immunoglobulin production. Science 2002; 298: 1630–1634.
Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ et al. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol 2005; 175: 2167–2173.
Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res 2003; 63: 9016–9022.
Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 2008; 111: 5326–5333.
Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res 2007; 13: 3630–3636.
Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol 2008; 26: 2034–2039.
Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK et al. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother 2008; 57: 1439–1449.
Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res 2009; 15: 2123–2129.
Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007; 448: 480–483.
Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007; 448: 484–487.
Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007; 8: 967–974.
Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 2004; 173: 5361–5371.
Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse III HC et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB- Yaa mice. Proc Natl Acad Sci USA 2009; 106: 1518–1523.
Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ . IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci USA 2008; 105: 14028–14033.
Sutherland AP, Van Belle T, Wurster AL, Suto A, Michaud M, Zhang D et al. IL-21 is required for the development of type 1 diabetes in NOD mice. Diabetes 2009; 58: 1144–1155.
Teshima T, Hill GR, Pan L, Brinson YS, van den Brink MR, Cooke KR et al. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J Clin Invest 1999; 104: 317–325.
Cooke KR, Gerbitz A, Crawford JM, Teshima T, Hill GR, Tesolin A et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest 2001; 107 (12): 1581–1589.
Reddy P, Teshima T, Hildebrandt G, Duffner U, Maeda Y, Cooke KR et al. 2002. Interleukin 18 preserves a perforin-dependent graft-versus-leukemia effect after allogeneic bone marrow transplantation. Blood 2002; 100: 3429–3431.
Yang YG, Qi J, Wang MG, Sykes M . Donor-derived interferon gamma separates graft-versus-leukemia effects and graft-versus-host disease induced by donor CD8T cells. Blood 2002; 99: 4207–4215.
Clouthier SG, Cooke KR, Teshima T, Lowler KP, Liu C, Connolly K et al. Repifermin (keratinocyte growth factor-2) reduces the severity of graft-versus-host disease while preserving a graft-versus-leukemia effect. Biol Blood Marrow Transplant 2003; 9: 592–603.
Reddy P, Maeda Y, Hotary K, Liu C, Reznikov LL, Dinarello CA et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci USA 2004; 101: 3921–3926.
Zhang C, Lou J, Li N, Todorov I, Lin CL, Cao YA et al. Donor CD8+ T cells mediate graft-versus-leukemia activity without clinical signs of graft-versus-host disease in recipients conditioned with anti-CD3 monoclonal antibody. J Immunol 2007; 178: 838–850.
Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 2003; 9: 1144–1150.
Meguro A, Ozaki K, Oh I, Hatanaka K, Matsu H, Tatara R et al. IL-21 is critical for GVHD in a mouse model. Bone Marrow Transplant 2010; 45: 723–729.
Bucher C, Koch L, Vogtenhuber C, Goren E, Munger M, Panoskaltsis-Mortari A et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood 2009; 114: 5375–5384.
Williams RT, Roussel MF, Sherr CJ . Arf gene loss enhances oncogenicity and limits imatinib response in mouse models of Bcr-Abl-induced acute lymphoblastic leukemia. Proc Natl Acad Sci USA 2006; 103: 6688–6693.
Oh I, Ozaki K, Meguro A, Hatanaka K, Kadowaki M, Matsu H et al. Altered effector CD4+ T cell function in IL-21R−/− CD4+ T cell-mediated graft-versus-host disease. J Immunol 2010; 185: 1920–1926.
Acknowledgements
We thank Dr Tanabe (Institute of Medical Science, University of Tokyo, Tokyo) for donating the bcr-abl vector, Dr Kitamura (Institute of Medical Science, University of Tokyo, Tokyo) for donating PLAT-E, a packaging cell line and Drs Miyoshi (Tsukuba Institute, RIKEN, Tsukuba) and Okano (Keio University, Tokyo) for donating the luciferase-containing lentivirus vector. This work was supported in part by grants from the Ministry of Health, Labor and Welfare of Japan, by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the Intramural Research Program of the National Heart, Lung and Blood Institute, National Institutes of Health (Bethesda, MD) and by a Young Investigator Award from Jichi Medical University, Tochigi, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Meguro, A., Ozaki, K., Hatanaka, K. et al. Lack of IL-21 signal attenuates graft-versus-leukemia effect in the absence of CD8 T-cells. Bone Marrow Transplant 46, 1557–1565 (2011). https://doi.org/10.1038/bmt.2010.342
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.342
Keywords
This article is cited by
-
Translational opportunities for targeting the Th17 axis in acute graft-vs.-host disease
Mucosal Immunology (2016)
-
IL-21 accelerates xenogeneic graft-versus-host disease correlated with increased B-cell proliferation
Protein & Cell (2013)
-
Advances in graft-versus-host disease biology and therapy
Nature Reviews Immunology (2012)