Abstract
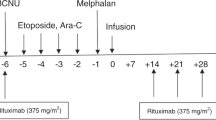
BEAM is a widely used conditioning regimen for relapsed/refractory lymphoma patients undergoing auto-SCT. We conducted a multicenter study with an alternative regimen (fotemustine plus etoposide, cytarabine and melphalan (FEAM)) in which BCNU was substituted by the chloroethylnitrosourea fotemustine (FTM). Eighty-four patients with relapsed/refractory Hodgkin's (n=20) and non-Hodgkin's lymphoma (n=64) were conditioned with a FEAM regimen (FTM 150 mg/m2 on days –7, –6, etoposide 200 mg/m2 and cytarabine 400 mg/m2 on days –5, –4, –3, –2 and melphalan 140 mg/m2 on day –1). Patients were evaluated for toxicity and engraftment parameters. Median times to neutrophil (>500 × 109/l) and plt (>20 000 × 109/l) engraftment were 11 and 13 days, respectively. Grade 3 mucositis occurred in 19 patients (23%), while G3 nausea/vomiting and G3 diarrhea were observed in 13 (15%) and 6 (7%) patients, respectively. No severe hepatic, renal or pulmonary toxicity was detected. Seven patients (7%) experienced G4 mucositis, while no other G4 toxicities or unexpected adverse events of any grade were recorded. Transplant-related mortality was 2.4%. We conclude that a FEAM regimen is feasible and safe. Although toxicity and engraftment times compared favorably with BEAM, longer follow-up is needed to evaluate fully its efficacy and long-term safety.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH . BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol 1995; 13: 588–595.
Jo JC, Kang BW, Jang G, Sym SJ, Lee SS, Koo JE et al. BEAC or BEAM high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin's lymphoma patients: comparative analysis of efficacy and toxicity. Ann Hematol 2008; 87: 43–48.
Jantunen E, Kuittinen T, Nousiainen T . BEAC or BEAM for high-dose therapy in patients with non-Hodgkin's lymphoma? A single centre analysis on toxicity and efficacy. Leuk Lymphoma 2003; 44: 1151–1158.
Salar A, Sierra J, Gandarillas M, Caballero MD, Marín J, Lahuerta JJ et al. GEL/TAMO Spanish Cooperative Group. Autologous stem cell transplantation for clinically aggressive non-Hodgkin's lymphoma: the role of preparative regimens. Bone Marrow Transplant 2001; 27: 405–412.
Alessandrino EP, Bernasconi P, Colombo A, Caldera D, Martinelli G, Vitulo P et al. Pulmonary toxicity following carmustine-based preparative regimens and autologous peripheral blood progenitor cell transplantation in hematological malignancies. Bone Marrow Transplant 2000; 25: 309–313.
Kehrer JP . The effect of BCNU (carmustine) on tissue glutathione reductase activity. Toxicol Lett 1983; 17: 63–68.
Cao TM, Negrin RS, Stockerl-Goldstein KE, Johnston LJ, Shizuru JA, Taylor TL et al. Pulmonary toxicity syndrome in breast cancer patients undergoing BCNU-containing high-dose chemotherapy and autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 2000; 6: 387–394.
Hayes MT, Bartley J, Parsons PG, Eaglesham GK, Prakash AS . Mechanism of action of fotemustine, a new chloroethylnitrosourea anticancer agent: evidence for the formation of two DNA-reactive intermediates contributing to cytotoxicity. Biochemistry 1997; 36: 10646–10654.
Levin VA . Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J Med Chem 1980; 23: 682–684.
Meulemans A, Giroux B, Hannoun P, Robine D, Henzel D . Comparative diffusion study of two nitrosoureas: carmustine and fotemustine in normal rat brain, human, and rat brain biopsies. Chemotherapy 1991; 37: 86–92.
Meulemans A, Giroux B, Hannoun P, Henzel D, Bizzari JP, Mohler J . Permeability of two nitrosoureas, carmustine and fotemustine in rat cortex. Chemotherapy 1989; 35: 313–319.
Guaitani A, Corada M, Lucas C, Lemoine A, Garattini S, Bartosek I . Pharmacokinetics of fotemustine and BCNU in plasma, liver and tumor tissue of rats bearing two lines of Walker 256 carcinoma. Cancer Chemother Pharmacol 1991; 28: 293–297.
Boutin JA, Norbeck K, Moldeus P, Genton A, Paraire M, Bizzari JP et al. Effects of the new nitrosourea derivative, fotemustine, on the glutathione reductase activity in rat tissues in vivo and in isolated rat hepatocytes. Eur J Cancer Clin Oncol 1989; 25: 1311–1316.
Brandes AA, Tosoni A, Franceschi E, Blatt V, Santoro A, Faedi M et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemother Pharmacol 2009; 64: 769–775 .
Fabrini MG, Silvano G, Lolli I, Perrone F, Marsella A, Scotti V et al. A multi-institutional phase II study on second-line fotemustine chemotherapy in recurrent glioblastoma. J Neurooncol 2009; 92: 79–86.
Scoccianti S, Detti B, Sardaro A, Iannalfi A, Meattini I, Leonulli BG et al. Second-line chemotherapy with fotemustine in temozolomide-pretreated patients with relapsing glioblastoma: a single institution experience. Anticancer Drugs 2008; 19: 613–620.
Jacquillat C, Khayat D, Weil M, Bizzari JP . Clinical phase I–II study of the nitrosourea servier 10036 (fotemustine) in hematological malignancies. Proc ECCO 1987; 4: 314.
Rigal-Huguet F, Gaspard MH, Attal M, Giroux B, Lucas C, Pris J . Clinical pharmacokinetics of high doses of fotemustine with assessment of passage across the blood-brain barrier. Bull Cancer 1990; 77: 599.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244–1253.
Schmitz N, Buske C, Gisselbrecht C . Autologous stem cell transplantation in lymphoma. Semin Hematol 2007; 44: 234–245.
Brice P . Managing relapsed and refractory Hodgkin lymphoma. Br J Haematol 2008; 141: 3–13.
Sirohi B, Cunningham D, Powles R, Murphy F, Arkenau T, Norman A et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma. Ann Oncol 2008; 19: 1312–1319.
Seshadri T, Kuruvilla J, Crump M, Keating A . Salvage therapy for relapsed/refractory diffuse large B cell lymphoma. Biol Blood Marrow Transplant 2008; 14: 259–267.
Hamlin PA, Zelenetz AD, Kewalramani T, Qin J, Satagopan JM, Verbel D et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood 2003; 102: 1989–1996.
Fernandez HF, Escalón MP, Pereira D, Lazarus HM . Autotransplant conditioning regimens for aggressive lymphoma: are we on the right road? Bone Marrow Transplant 2007; 40: 505–513.
Gisselbrecht C . Use of rituximab in diffuse large B-cell lymphoma in the salvage setting. Br J Haematol 2008; 143: 607–621.
Zhang MM, Gopal AK . Radioimmunotherapy-based conditioning regimens for stem cell transplantation. Semin Hematol 2008; 45: 118–125.
Caballero MD, Rubio V, Rifon J, Heras I, García-Sanz R, Vázquez L et al. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant 1997; 20: 451–458.
van Besien K, Tabocoff J, Rodriguez M, Andersson B, Mehra R, Przepiorka D et al. High-dose chemotherapy with BEAC regimen and autologous bone marrow transplantation for intermediate grade and immunoblastic lymphoma: durable complete remissions, but a high rate of regimen-related toxicity. Bone Marrow Transplant 1995; 15: 549–555.
Seiden MV, Elias A, Ayash L, Hunt M, Eder JP, Schnipper LE et al. Pulmonary toxicity associated with high dose chemotherapy in the treatment of solid tumors with autologous marrow transplant: an analysis of four chemotherapy regimen. Bone Marrow Transplant 1992; 10: 57–63.
Phillips GL, Fay JW, Herzig GP, Herzig RH, Weiner RS, Wolff SN et al. Intensive 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), NSC 436650 and cryopreserved autologous marrow transplantation for refractory cancer: a phase I–II study. Cancer 1983; 52: 1792–1802.
De Rossi A, Rossi L, Laudisi A, Sini V, Toppo L, Marchesi F et al. Focus on fotemustine. J Exp Clin Cancer Res 2006; 25: 461–468.
Laquerriere A, Raguenez-Viotte G, Paraire M, Bizzari JP, Paresy M, Fillastre JP et al. Nitrosoureas lomustine, carmustine and fotemustine induced hepatotoxic perturbations in rats: biochemical. Morphological and flow cytometry studies. Eur J Cancer 1991; 27: 630–638.
Lee SM, Thatcher N, Dougal M, Margison GP . Dosage and cycle effects of dacarbazine (DTIC) and fotemustine on O6-alkylguanine-DNA alkyltransferase in human peripheral blood mononuclear cells. Br J Cancer 1993; 67: 216–221.
Briegert M, Kaina B . Human monocytes, but not dendritic cells derived from them, are defective in base excision repair and hypersensitive to methylating agents. Cancer Res 2007; 67: 26–31.
Filippeschi S, Colombo T, Bassani D, De Francesco L, Arioli P, D’Incalci M et al. Antitumor activity of the novel nitrosourea S10036 in rodent tumors. Anticancer Res 1988; 8: 1351–1354.
Khayat D, Lokiec F, Bizzari JP, Weil M, Meeus L, Sellami M et al. Phase I clinical study of the new amino acid-linked nitrosourea, S 10036, administered on a weekly schedule. Cancer Res 1987; 47: 6782–6785.
Dumontet C, Jaubert J, Sebban C, Bouafia F, Ardiet C, Tranchand B et al. Clinical and pharmacokinetic phase II study of fotemustine in refractory and relapsing multiple myeloma patients. Ann Oncol 2003; 14: 615–622.
Mangiacavalli S, Pica G, Varettoni M, Lazzarino M, Corso A . Efficacy and safety of fotemustine for the treatment of relapsed and refractory multiple myeloma patients. Eur J Haematol 2009; 82: 240–241.
Escalón MP, Stefanovic A, Venkatraman A, Pereira D, Santos ES, Goodman M et al. Autologous transplantation for relapsed non-Hodgkin's lymphoma using intravenous busulfan and cyclophosphamide as conditioning regimen: a single center experience. Bone Marrow Transplant 2009; 44: 89–96 .
Puig N, de la Rubia J, Remigia MJ, Jarque I, Martín G, Cupelli L et al. Morbidity and transplant-related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem-cell transplantation. Leuk Lymphoma 2006; 47: 1488–1494.
Blijlevens N, Schwenkglenks M, Bacon P, D’Addio A, Einsele H, Maertens J et al. European Blood and Marrow Transplantation Mucositis Advisory Group. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM in patients receiving chemotherapy-European blood and marrow transplantation mucositis advisory group. J Clin Oncol 2008; 26: 1519–1525.
Acknowledgements
We thank the following physicians who participated in the study: Rosella Matera, Ospedale Vito Fazi, Lecce, Potito Rosario Scalzulli, Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG), Paolo Di Carlo, Ospedale Civile, Pescara. This work was supported in part by a grant to A Pinto from Ministero della Salute, Ricerca Finalizzata FSN, IRCCS, Rome, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Tania Perrone is an employee of Italfarmaco S.p.A., Italy. All other authors have no financial or other conflict to declare.
Rights and permissions
About this article
Cite this article
Musso, M., Scalone, R., Marcacci, G. et al. Fotemustine plus etoposide, cytarabine and melphalan (FEAM) as a new conditioning regimen for lymphoma patients undergoing auto-SCT: a multicenter feasibility study. Bone Marrow Transplant 45, 1147–1153 (2010). https://doi.org/10.1038/bmt.2009.318
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.318
Keywords
This article is cited by
-
The role of stem cell transplantation in the management of relapsed follicular lymphoma in the era of targeted therapies
Bone Marrow Transplantation (2019)
-
Modified BEAM as conditioning regimen for lymphoma patients undergoing autologous hematopoietic stem cell transplantation
Bone Marrow Transplantation (2018)
-
BEAM vs FEAM high-dose chemotherapy: retrospective study in lymphoma patients undergoing autologous stem cell transplant
Bone Marrow Transplantation (2018)
-
A retrospective comparison of toxicity and initial efficacy of two autologous stem cell transplant conditioning regimens for relapsed lymphoma: LEAM and BEAM
Bone Marrow Transplantation (2016)