Abstract
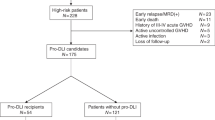
This study examines the absolute numbers and relative proportions of CD4+, CD8+, CD14+ and CD34+ cells contained in allografts and their impact on early engraftment and later clinical outcomes in 141 patients with hematological malignancies who underwent unmanipulated HLA-mismatched/haploidentical hematopoietic SCT without in vitro T-cell depletion. These patients received G-CSF-primed BM grafts (G-BM) and peripheral blood grafts (G-PB) following a modified regimen of BU/CY 2 plus antithymocyte globulin. Multivariate analysis showed that high CD34+ cell numbers were associated with accelerated plt engraftment (P=0.001). Meanwhile, patients with a higher CD4/CD8 ratio in G-BM (⩾1.16) had a survival disadvantage (P<0.01) and a trend towards relapse (P=0.086) after controlling for disease status. A higher CD4/CD8 was also associated with a significantly increased risk of acute GVHD grades II–IV (P=0.013), even after adjusting for an ABO major mismatch. No aspect of graft composition affected neutrophil engraftment or chronic GVHD. In conclusion, the differences in CD34+ cell dose and the CD4/CD8 ratio in grafts seem to affect engraftment and clinical outcomes; in particular, a lower CD4/CD8 ratio in primed BM graft is associated with a survival benefit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mohty M, Bilger K, Jourdan E, Kuentz M, Michallet M, Bourhis JH et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia 2003; 17: 869–875.
Zaucha JM, Gooley T, Bensinger WI, Heimfeld S, Chauncey TR, Zaucha R et al. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood 2001; 98: 3221–3227.
Mavroudis D, Read E, Cottler-Fox M, Couriel D, Molldrem J, Carter C et al. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood 1996; 88: 3223–3229.
Kernan NA, Collins NH, Juliano L, Cartagena T, Dupont B, O'Reilly RJ . Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood 1986; 68: 770–773.
Cao TM, Shizuru JA, Wong RM, Sheehan K, Laport GG, Stockerl-Goldstein KE et al. Engraftment and survival following reduced-intensity allogeneic peripheral blood hematopoietic cell transplantation is affected by CD8+ T-cell dose. Blood 2005; 105: 2300–2306.
Tanaka J, Mielcarek M, Torok-Storb B . Impaired induction of the CD28-responsive complex in granulocyte colony-stimulating factor mobilized CD4 T cells. Blood 1998; 91: 347–352.
Zaucha JM, Gooley T, Heimfeld S . The ratio of CD3: CD14 cells and the number of CD34 cells in G-CSF mobilized peripheral blood mononuclear cell (G-PBMC) products are significantly associated with clinical outcome in HLA-identical sibling transplantation. Blood 2000; 96 (Suppl 1): 205a.
Morton J, Hutchins C, Durrant S . Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: significantly less graft-versus-host disease and comparable engraftment to G-CSF-mobilized peripheral blood stem cells. Blood 2001; 98: 3186–3191.
Ji SQ, Chen HR, Wang HX, Yan HM, Pan SP, Xun CQ . Comparison of outcome of allogeneic bone marrow transplantation with and without granulocyte colony-stimulating factor (lenograstim) donor-marrow priming in patients with chronic myelogenous leukemia. Biol Blood Marrow Transplant 2002; 8: 261–267.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006; 38: 291–297.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107: 3065–3073.
Dey BR, Spitzer TR . Current status of haploidentical stem cell transplantation. Br J Haematol 2006; 135: 423–437.
Tabilio A, Falzetti F, Zei T, De Ioanni M, Bonifacio E, Battelli F et al. Graft engineering for allogeneic haploidentical stem cell transplantation. Blood Cells Mol Dis 2004; 33: 274–280.
Chen SH, Li X, Huang XJ . Effect of recombinant human granulocyte colony-stimulating factor on T-lymphocyte function and the mechanism of this effect. Int J Hematol 2004; 79: 178–184.
Jun HX, Jun CY, Yu ZX . In vivo induction of T-cell hyporesponsiveness and alteration of immunological cells of bone marrow grafts using granulocyte colony-stimulating factor. Haematologica 2004; 89: 1517–1524.
Huang XJ, Chang YJ, Zhao XY . Maintaining hyporesponsiveness and polarization potential of T cells after in vitro mixture of G-CSF mobilized peripheral blood grafts and G-CSF primed bone marrow grafts in different proportions. Transpl Immunol 2007; 17: 193–197.
Liu Y, Chen S, Yu H . Standardization and Quality Control in Flow Cytometric Enumeration of CD34(+) cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2000; 8: 302–306.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204.
Katz MH . Multivariable Analysis: A Practical Guide for Clinicians, 2nd edn. Cambridge University Press: Cambridge, 2006.
Gray RJ . A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154.
Singhal S, Powles R, Treleaven J, Kulkarni S, Sirohi B, Horton C et al. A low CD34+ cell dose results in higher mortality and poorer survival after blood or marrow stem cell transplantation from HLA-identical siblings: should 2 × 10(6) CD34+ cells/kg be considered the minimum threshold? Bone Marrow Transplant 2000; 26: 489–496.
Slowman S, Danielson C, Graves V, Kotylo P, Broun R, McCarthy L . Administration of GM-/G-CSF prior to bone marrow harvest increases collection of CD34+ cells. Prog Clin Biol Res 1994; 389: 363–369.
Anasetti C, Heimfeld S, Rowley S, Martin PJ, Petersdorf EW, Woolfrey A et al. Higher CD34 cell dose is associated with improved survival after marrow transplantation from unrelated donors. Blood 1999; 94 (S1): 2509a.
Morariu-Zamfir R, Rocha V, Devergie A, Socie G, Ribaud P, Esperou H et al. Influence of CD34(+) marrow cell dose on outcome of HLA-identical sibling allogeneic bone marrow transplants in patients with chronic myeloid leukaemia. Bone Marrow Transplant 2001; 27: 575–580.
Pavletic ZS, Bishop MR, Tarantolo SR, Martin-Algarra S, Bierman PJ, Vose JM et al. Hematopoietic recovery after allogeneic blood stem-cell transplantation compared with bone marrow transplantation in patients with hematologic malignancies. J Clin Oncol 1997; 15: 1608–1616.
Lickliter JD, McGlave PB, DeFor TE, Miller JS, Ramsay NK, Verfaillie CM et al. Matched-pair analysis of peripheral blood stem cells compared to marrow for allogeneic transplantation. Bone Marrow Transplant 2000; 26: 723–728.
Zaucha JM, Zellmer E, Georges G, Little MT, Storb R, Storer B et al. G-CSF-mobilized peripheral blood mononuclear cells added to marrow facilitates engraftment in nonmyeloablated canine recipients: CD3 cells are required. Biol Blood Marrow Transplant 2001; 7: 613–619.
Bacigalupo A, Van Lint MT, Occhini D, Margiocco M, Ferrari G, Pittaluga PA et al. ABO compatibility and acute graft-versus-host disease following allogeneic bone marrow transplantation. Transplantation 1988; 45: 1091–1094.
Stussi G, Muntwyler J, Passweg JR, Seebach L, Schanz U, Gmur J et al. Consequences of ABO incompatibility in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 30: 87–93.
Martins SLR, St. John LS, Champlin RE, Wieder ED, McMannis J, Molldrem JJ et al. Functional assessment and specific depletion of alloreactive human T cells using flow cytometry. Blood 2004; 104: 3429–3436.
Yakoub-Agha I, Saule P, Depil S, Micol JB, Grutzmacher C, Boulanger-Villard F et al. A high proportion of donor CD4+ T cells expressing the lymph node-homing chemokine receptor CCR7 increases incidence and severity of acute graft-versus-host disease in patients undergoing allogeneic stem cell transplantation for hematological malignancy. Leukemia 2006; 20: 1557–1565.
Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood 2006; 108: 1291–1297.
Higman MA, Vogelsang GB . Chronic graft versus host disease. Br J Haematol 2004; 125: 435–454.
Horwitz ME, Sullivan KM . Chronic graft-versus-host disease. Blood Rev 2006; 20: 15–27.
Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 2005; 106: 2903–2911.
van den Brink MRM, Moore E, Ferrara JLM, Burakoff SJ . Graft-versus-host-disease-associated thymic damage results in the appearance of T cell clones with anti-host reactivity. Transplantation 2000; 69: 446–449.
Dong L, Wu T, Zhang MJ, Gao ZY, Lu DP . CD3+ cell dose and disease status are important factors determining clinical outcomes in patients undergoing unmanipulated haploidentical blood and marrow transplantation after conditioning including antithymocyte globulin. Biol Blood Marrow Transplant 2007; 13: 1515–1524.
Acknowledgements
This work was supported by the Hi-Tech Research and Development Program of China (No. 2006AA02Z4A0), and the China National Funds for Distinguished Young Scientist's (Grant no. 30725038), and the Funds for Creative Research Groups of China (IRT0702). We thank San Francisco Edit (www.sefedit.net) for their assistance in editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)
Supplementary information
Rights and permissions
About this article
Cite this article
Luo, XH., Chang, YJ., Xu, LP. et al. The impact of graft composition on clinical outcomes in unmanipulated HLA-mismatched/haploidentical hematopoietic SCT. Bone Marrow Transplant 43, 29–36 (2009). https://doi.org/10.1038/bmt.2008.267
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.267
Keywords
This article is cited by
-
Exploring strategies to optimise outcomes in hepatitis-associated aplastic anaemia patients following haematopoietic stem cell transplantation
Scientific Reports (2024)
-
Contemporary haploidentical stem cell transplant strategies in children with hematological malignancies
Bone Marrow Transplantation (2021)
-
Seeking biomarkers for acute graft-versus-host disease: where we are and where we are heading?
Biomarker Research (2019)
-
Everyone has a donor: contribution of the Chinese experience to global practice of haploidentical hematopoietic stem cell transplantation
Frontiers of Medicine (2019)
-
Comparison of efficacy between HLA6/6- and HLA3/6-matched haploidentical hematopoietic stem cell transplant in T-cell-replete transplants between parents and children
Science China Life Sciences (2019)