Abstract
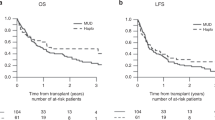
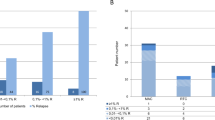
Lineage-specific chimerism studies are commonly obtained at several time points after nonmyeloablative hematopoietic cell transplantation to assess the tempo and degree of engraftment, and to monitor graft rejection. For patients who receive myeloablative transplants, the value of frequent chimerism analyses using sensitive molecular techniques is less certain. In this study, a retrospective analysis was performed to assess the transplant outcome of 89 adult patients with ALL who had chimerism studies of unfractionated BM cells or peripheral blood subsets performed approximately 80 days after transplantation. These patients received unmanipulated, myeloablative transplants using either HLA-identical or HLA-mismatched, related or unrelated donor stem cells. Incomplete donor engraftment was present only in the CD3+ peripheral blood T cells in a small percentage of patients. There was no correlation of mixed chimerism with transplant outcome. Routine ‘day 80’ chimerism studies in this group of patients who receive intensive, myeloablative conditioning regimens are not recommended.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Antin JH, Childs R, Filipovich AH, Giralt S, Mackinnon S, Spitzer T et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings (Review). Biol Blood Marrow Transplant 2001; 7: 473–485.
Baron F, Sandmaier BM . Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning (Review). Leukemia 2006; 20: 1690–1700.
Petersdorf EW, Gooley TA, Anasetti C, Martin PJ, Smith AG, Mickelson EM et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood 1998; 92: 3515–3520.
Storb R, Deeg HJ, Pepe M, Appelbaum FR, Anasetti C, Beatty P et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood 1989; 73: 1729–1734.
Dewald GW, Schad CR, Christensen ER, Law ME, Zinsmeister AR, Stalboerger PG et al. Fluorescence in situ hybridization with X and Y chromosome probes for cytogenetic studies on bone marrow cells after opposite sex transplantation. Bone Marrow Transplant 1993; 12: 149–154.
Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA . Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood 1995; 85: 1954–1963.
Smith AG, Martin PJ . Analysis of amplified variable number tandem repeat loci for evaluation of engraftment after hematopoietic stem cell transplantation. Rev Immunogenet 1999; 1: 255–264.
Thiede C, Bornhauser M, Oelschlagel U, Brendel C, Leo R, Daxberger H et al. Sequential monitoring of chimerism and detection of minimal residual disease after allogeneic blood stem cell transplantation (BSCT) using multiplex PCR amplification of short tandem repeat-markers. Leukemia 2001; 15: 293–302.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol 2004; 22: 1696–1705.
Bryant E, Martin PJ . Documentation of engraftment and characterization of chimerism following hematopoietic cell transplantation. In: Blume KG, Forman SJ, Appelbaum FR (eds). Thomas’ Hematopoietic Cell Transplantation. Blackwell: Oxford, UK, 2004, pp 234–243.
Doney K, Hägglund H, Leisenring W, Chauncey T, Appelbaum FR, Storb R . Predictive factors for outcome of allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2003; 9: 472–481.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doney, K., Loken, M., Bryant, E. et al. Lack of utility of chimerism studies obtained 2–3 months after myeloablative hematopoietic cell transplantation for ALL. Bone Marrow Transplant 42, 271–274 (2008). https://doi.org/10.1038/bmt.2008.155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.155
Keywords
This article is cited by
-
Long-term survival with mixed chimerism in patients with AML and MDS transplanted after conditioning with targeted busulfan, fludarabine, and thymoglobulin
Bone Marrow Transplantation (2022)
-
Chimerism analysis for clinicians: a review of the literature and worldwide practices
Bone Marrow Transplantation (2022)
-
Monitoring minimal residual/relapsing disease after allogeneic haematopoietic stem cell transplantation in adult patients with acute lymphoblastic leukaemia
Bone Marrow Transplantation (2020)
-
Impact of early chimerism status on clinical outcome in children with acute lymphoblastic leukaemia after haematopoietic stem cell transplantation
BMC Cancer (2019)
-
Early recipient chimerism testing in the T- and NK-cell lineages for risk assessment of graft rejection in pediatric patients undergoing allogeneic stem cell transplantation
Leukemia (2012)