Abstract
Background:
EWSR1 rearrangements were first identified in Ewing sarcoma, but the spectrum of EWSR1-rearranged neoplasms now includes many soft tissue tumour subtypes including desmoplastic small round cell tumour (DSRCT), myxoid liposarcoma (MLPS), extraskeletal myxoid chondrosarcoma (EMC), angiomatoid fibrous histiocytoma (AFH), clear cell sarcoma (CCS) and myoepithelial neoplasms. We analysed the spectrum of EWSR1-rearranged soft tissue neoplasms at our tertiary sarcoma centre, by assessing ancillary molecular diagnostic modalities identifying EWSR1-rearranged tumours and reviewing the results in light of our current knowledge of these and other Ewing sarcoma-like neoplasms.
Methods:
We retrospectively analysed all specimens tested for EWSR1 rearrangements by fluorescence in situ hybridisation (FISH) and/or reverse transcription–PCR (RT–PCR) over a 7-year period.
Results:
There was a total of 772 specimens. FISH was performed more often than RT–PCR (n=753, 97.5% vs n=445, 57.6%). In total, 210 (27.9%) specimens were FISH-positive for EWSR1 rearrangement compared to 111 (14.4%) that showed EWSR1 fusion transcripts with RT–PCR. Failure rates for FISH and RT–PCR were 2.5% and 18.0%. Of 109 round cell tumours with pathology consistent with Ewing sarcoma, 15 (13.8 %) cases were FISH-positive without an identifiable EWSR1 fusion transcript, 4 (3.7%) were FISH-negative but RT–PCR positive and 4 (3.7%) were negative for both. FISH positivity for DSRCT, MLPS, EMC, AFH and CCS was 86.3%, 4.3%, 58.5%, 60.0% and 87.9%, respectively. A positive FISH result led to diagnostic change in 40 (19.0%) EWSR1-rearranged cases. 13 FISH-positive cases remained unclassifiable.
Conclusions:
FISH is more sensitive for identifying EWSR1 rearrangements than RT–PCR. However, there can be significant morphologic and immunohistochemical overlap between groups of EWSR1-rearranged neoplasms, with important prognostic and therapeutic implications. FISH and RT–PCR should be used as complementary modalities in diagnosing EWSR1-rearranged neoplasms, but as tumour groups harbouring EWSR1 rearrangements are increasingly characterised and because given translocations involving EWSR1 and its partner genes are not always specific for tumour types, it is critical that these are evaluated by specialist soft tissue surgical pathologists noting the morphologic and immunohistochemical context. As RT–PCR using commercial primers is limited to only the most prevalent EWSR1 fusion transcripts, the incorporation of high-throughput sequencing technologies into the standard diagnostic repertoire to assess for multiple molecular abnormalities of soft tissue tumours in parallel (including detection of newly characterised Ewing sarcoma-like tumours) might be the most effective and efficient means of ancillary diagnosis in future.
Similar content being viewed by others
Main
Soft tissue neoplasms are a heterogeneous group unified only by their differentiation towards various mesenchymal lineages. Their classification is incomplete and continues to be refined, aided particularly by several recent molecular advances. A proportion of soft tissue tumours harbours characteristic, reproducible genetic abnormalities, including chromosomal translocations that result in the fusion of two separate genes, of which there are almost 100 uniquely identified in sarcoma (Mertens and Tayebwa, 2014); molecular techniques are therefore a crucial and routine adjunct to diagnosis. First observed to be rearranged in Ewing sarcoma (Aurias et al, 1983), the Ewing sarcoma breakpoint region 1 gene (EWSR1) on chromosome 22q12 encodes a 656 amino acid nuclear protein including a carboxy-terminus 87-amino acid RNA-binding domain (exons 11–13) involved in protein–RNA binding, transcription and RNA metabolism (Fisher, 2014). Its role in cancer cell progression is still unclear, although it may play a critical role in DNA damage response and cell division (Li et al, 2007; Paronetto, 2013). While initially thought specific for Ewing sarcoma (formerly the Ewing sarcoma/primitive peripheral neuroectodermal tumour (PNET) family of tumours) (Dockhorn Dworniczak et al, 1994), characteristic rearrangements between EWSR1 and partner genes have been documented in both tumours of mesenchymal and non-mesenchymal lineage, including desmoplastic small round cell tumour (DSRCT; Ladanyi and Gerald, 1994; Antonescu et al, 1998), myxoid liposarcoma (MLPS; Panagopoulos et al, 1996; Dal Cin et al, 1997; Hosaka et al, 2002), extraskeletal myxoid chondrosarcoma (EMC; Sciot et al, 1995; Clark et al, 1996), angiomatoid fibrous histiocytoma (AFH; Hallor et al, 2005; Rossi et al, 2007; Thway and Fisher, 2015; Thway et al, 2015b), clear cell sarcoma of soft tissue (CCS; Hisaoka et al, 2008; Wang et al, 2009) and clear cell sarcoma-like tumours of the gastrointestinal tract (CCSLGT; Thway and Fisher, 2012; Wang and Thway, 2015), primary pulmonary myxoid sarcoma (PPMS; Thway et al, 2011), myoepithelial tumours of skin, soft tissue and bone (Antonescu et al, 2010a; Antonescu et al, 2010b; Thway and Fisher, 2014; Thway et al, 2015a), and more rarely in low-grade fibromyxoid sarcoma (LGFMS; Lau et al, 2013) and sclerosing epithelioid fibrosarcoma (SEF; Doyle et al, 2012; Arbajian et al, 2014). EWSR1 rearrangements can be easily detected in the routine setting by fluorescence in situ hybridisation (FISH) with break-apart probes, and corresponding fusion transcripts by reverse transcription–PCR (RT–PCR) studies, usually using commercial probes and primers respectively. In view of the increasing prominence of EWSR1 rearrangement in soft tissue neoplasms, we evaluated the utility of FISH and RT–PCR as ancillary diagnostic tools in assessing potential EWSR1-rearranged neoplasms in today’s current practice, and assessed the spectrum of EWSR1-rearranged neoplasms at our tertiary centre over the course of the establishment of the ancillary molecular diagnostics and molecular cytogenetics services.
Materials and methods
All neoplasms which had FISH and/or RT–PCR performed to assess for EWSR1 rearrangement or for fusion transcripts containing EWSR1 were identified from 2008 to 2015 from the prospectively maintained Royal Marsden Hospital (RMH) molecular genetics and cytogenetics databases (DG and JS). Information regarding diagnoses, morphology and immunohistochemistry was found on the matched surgical pathology reports. Prior to EWSR1 rearrangement testing, diagnoses were made from morphology and immunohistochemistry by one or two (KT and CF) soft tissue specialist (consultant/attending) pathologists. Tumours with pathologic features in keeping with an EWSR1-rearranged neoplasm were tested to confirm diagnosis, as the presence of EWSR1 fusion transcripts, or of the presence of an EWSR1 rearrangement in the appropriate pathologic and clinical context, would represent the diagnostic gold standard; tumours with pathologic features suggestive of an EWSR1-rearranged neoplasm or in which an EWSR1-rearranged neoplasm could not be excluded were also tested. Finally, tumours that were difficult to classify morphologically and immunohistochemically that could conceivably represent atypical variants of EWSR1-rearranged neoplasms were also tested. FISH or RT–PCR tests were requested only by a consultant/attending soft tissue pathologist, and chosen in light of the clinical and pathologic picture and tissue availability. Further diagnostic interpretation was then made according to the results of ancillary molecular testing. All cases were formalin-fixed and paraffin-embedded (FFPE) and included both core biopsy and excision specimens of material biopsied or resected at our centre, or were external cases that had been sent for review or second opinion.
For FISH, 2- to 4-μm thick FFPE sections were dewaxed overnight at 60 °C, treated with hot buffer wash at 80 °C (2–3 h) then proteolytic enzyme treatment at 37 °C, and finally washed in distilled water and then an alcohol series before addition of an EWSR1 break-apart probe (Vysis, Abbott Laboratories Ltd, Maidenhead, UK). Hybridisation was performed overnight according to the manufacturer’s protocols. An extra pressure cooker step was more recently added, whereby 35 ml of antigen retrieval buffer and 3.5 l of distilled water were brought to a boil in a pressure cooker, the dewaxed slides added, the pressure raised and the cooker cooled after five minutes, after which the slides were washed twice in distilled water before proceeding to the hybridisation stage (Vroobel et al, 2016). After FISH, images of the sections were captured using a cooled charged-coupled device camera. To minimise nuclear truncation artefacts, only nuclei with at least two EWSR1 signals were evaluated. Overlapping tumour nuclei were also excluded from evaluation to decrease false-positive scoring. With this probe used in this study, separated signals that were at least three signal widths apart were scored as positive. However, occasional, scattered positive cells are not sufficient for a case to be classed as positive; laboratory policy is that there should be at least 5% of evaluable nuclei in at least three high-power fields, excluding fields with no positive cells present. Overall, a FISH study was classed as positive if two analysts were in agreement that the number of nuclei with clearly separated signals was sufficient without needing a formal count. In very rare cases, a very small number of abnormal cells was seen and then a third, usually more senior analyst would give an assessment and a formal count would be made. If the proportion of typically abnormal cells in three selected high-power fields constituted less than 5% of the assessable cells, then this would be either disregarded or it would be reported with a caveat to say that there were too few cells to support the diagnosis.
For reverse transcription real-time quantitative–PCR(RQ–PCR), RNA was extracted from FFPE samples using the RecoverAll Total Nucleic Acid Extraction kit (Ambion Ltd., Cambridgeshire, UK) and transcribed into complementary DNA (cDNA) using the High Capacity cDNA kit (Applied Biosystems, Warrington, UK) according to the manufacturers’ recommendations. Amplification of B2M was performed to assess the quality of the RNA as described previously (Thway et al, 2016). RQ–PCR reactions were performed to assess for any or all of the following fusion transcripts: EWSR1-NR4A3, TAF15-NR4A3, EWSR1-FLI1, EWSR1-ERG, EWSR1-WT1, EWSR1-ATF1 and EWSR1-CREB1). Primers and probe sequences were adapted from the literature (Okamoto et al, 2001; Antonescu et al, 2002; Jin et al, 2003; Hostein et al, 2004; Coindre et al, 2006; Antonescu et al, 2007; Lewis et al, 2007) (please see Supplementary Table A for the primer sequences of the tested fusion transcripts) RQ–PCR was performed on a 7500-Fast real-time PCR system (Applied Biosystems), using Universal TaqMan Master Mix (2X) (Applied Biosystems), 300 nM of each primer, 100 nM of probe, and 5 μl of cDNA in a total volume of 20 μl. Samples were run in duplicate together with a negative and positive control for each reaction.
Results
A total of 812 specimens from 762 patients were analysed for EWSR1 rearrangement by either FISH, RT–PCR or both modalities. After duplicate cases (repeat testing done on the same specimen) were excluded, 772 specimens were included in our analysis (Tables 1, 2, 3). Routine FISH was performed on 753 (97.5%) samples, of which 210 (27.9%) were positive for an EWSR1 rearrangement and 524 (69.6%) were negative. The FISH study failed in 19 (2.5%) cases. RT–PCR was less commonly used (445 cases, 57.6%). A fusion transcript containing an EWSR1 rearrangement was documented in 111 (24.9%) samples. Testing failed in 80 (18.0%) cases. Of the 210 FISH-positive cases, RT–PCR was performed in 174, and a fusion transcript was identified in 99/174 (56.9%) cases. Subsequent to a positive FISH result, the initial diagnosis based on morphology and immunohistochemistry was changed in 40 (19.0%) cases (Supplementary Table B). Most of these 40 represented diagnostically complex cases of neoplasms in which there was significant morphologic and immunophenotypic overlap with other tumours of other lineages or other sarcomas. Among these, 14 were initially thought to represent small cell carcinoma and were subsequently changed to a diagnosis of Ewing sarcoma or DSRCT. Other confounding diagnoses included differentiating melanoma from clear cell sarcoma and poorly differentiated synovial sarcoma (which has predominantly round cell morphology) from Ewing sarcoma. In five FISH-negative cases, a fusion transcript was identified and also led to a change in the initial diagnosis. EWSR1 rearrangement testing (FISH and/or RT–PCR) was performed in 125 undifferentiated neoplasms, of which 62 were composed of spindle cells, 26 of round cells, 7 of pleomorphic cells, with the remainder having mixed morphology. A FISH-positive result was documented in six (4.8%) cases (three spindle, one round and two of mixed morphology). The most common diagnoses for neoplasms that were doubly FISH and RT–PCR negative for EWSR1-rearrangements are shown in Supplementary Table C.
EWSR1-rearranged neoplasms
Results from FISH and RT–PCR studies routinely used for EWSR1-rearranged neoplasms are summarised in Tables 1, 2, 3. The great majority of cases suspected to represent an EWSR1-rearranged neoplasm underwent FISH analysis. Suspected cases of Ewing sarcoma, DRSCT, CCS, CCSLGT, PPMS, AFH and EMC were more likely to undergo RT–PCR testing compared to suspected cases of myoepithelial neoplasms and LGFMS. Positive concordance rates between FISH and RT–PCR studies varied between 34.1% and 80.0%. On the basis of morphology and immunohistochemistry, 109 cases were diagnosed as probable or possible Ewing sarcoma. In total, 89 (81.7%) cases had a positive FISH test (EWSR1 rearrangement) (Figure 1A) and 50 (58.1%) had identifiable EWSR1-FLI1 or EWSR1-ERG fusion transcripts (92.0% and 8.0%, respectively). EWSR1 fusion transcripts by RT–PCR were not found in 15 (13.8%) FISH-positive cases, with these likely representing rare variant fusions. Furthermore, 18 cases (16.5%) were FISH-negative; 4 (3.7%) had an identifiable fusion transcript and 4 (3.7%) did not; RT–PCR failed or was not performed (due to lack of sufficient material) in the remaining cases. The four cases which were morphologically and immunohistochemically thought to represent Ewing sarcoma but which were FISH and RT–PCR negative are shown in Table 4. All cases comprised hypercellular neoplasms, generally with prominent mitoses and necrosis, and 3/4 were composed of uniform or monotonous small, rounded cells with scanty cytoplasm, with one showing nuclei with moderate or sometimes marked atypia. All showed at least focal expression of CD99 and often of neural/ neuroectodermal markers and were negative for other markers including cytokeratins, desmin, CD34 and haematolymphoid markers. The final interpretations were of small round cell tumour, possibly Ewing sarcoma with variant partner gene, or in the case with cellular atypia, of possible atypical Ewing sarcoma. Clinically, these were all highly aggressive tumours; three of the four patients with follow up died of progressive or metastatic disease within 18 months of diagnosis. The fourth patient had chemotherapy and a below-knee amputation but was subsequently lost to follow up (Table 4). Among the 22 suspected cases of DRSCT, the FISH positivity rate was high (86.3%). RT–PCR reliably identified the EWSR1-WT1 transcript in 2 of 3 FISH-negative cases. High FISH positivity rates were also documented in CCS (87.9%) and CCSLGT (80.0%). A fusion transcript involving EWSR1-ATF1 or EWSR1-CREB1 was identified in all cases of CCSLGT diagnosed pathologically. An EWSR1 rearrangement was less prevalent in EMC, AFH, PPMS, myoepithelial neoplasms, LGFMS and SEF. As would be typical, no fusion transcript involving EWSR1-ATF1 or EWSR1-CREB1 was found in EWSR1 FISH-positive myoepithelial neoplasms. Among the EWSR1-negative samples, 29 samples were positive for a FUS rearrangement either by FISH or RT–PCR: 18 cases were diagnosed as myxoid liposarcoma, 9 cases as LGFMS and 2 cases as SEF.
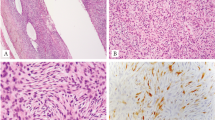
Fluorescence in situ hybridisation for EWSR1 gene rearrangement, and histology of variant Ewing sarcoma types. (A) Fluorescence in situ hybridisation using dual-colour break-apart probes which flank the EWSR1 breakpoint region on chromosome 22q12. The nucleus of this neoplasm contains separated (split) red and green signals indicating a rearrangement involving the EWSR1 gene at 22q12. A fused normal signal is also present, denoting the site of the EWSR1 gene. (B and C) Ewing sarcoma. These are examples of Ewing sarcomas with morphologic features that can cause diagnostic difficulty, particularly as they can show immunophenotypical overlap with other round cell neoplasms. (B) Ewing sarcoma with a well-defined nested architecture and areas of cellular discohesion mimicking alveolar rhabdomyosarcoma; (C) Ewing sarcoma with irregular cellular nests in prominent desmoplastic stroma, mimicking desmoplastic small round cell tumour, and (D) large cell Ewing sarcoma. RT–PCR in each case was diagnostically contributory, as this showed the presence of EWSR1-FLI1 fusion transcripts diagnostic of Ewing sarcoma.
Reliability of FISH and RT–PCR
FISH was the more reliable ancillary diagnostic test, with a failure rate for FISH of 2.5% compared with 18.0% for RT–PCR. FISH failure rates remained relatively constant from 2008 through 2015 and were 2.6%, 1.6%, 3.0%, 0.0%, 1.1%, 2.3%, 4.6% and 0.0%, respectively. RT–PCR failure rates were much higher (29.3%, 36.4%, 23.5%, 4.2%, 6.7%, 15.1%, 11.6%, 15.3%, respectively) but did improve over time. FISH was most likely to fail in myoepithelial neoplasms (7.1%) and AFH (5.0%) compared to other EWSR1-rearranged neoplasms. The RT–PCR failure rate was highest for Ewing sarcoma (19.8%), DRSCT (20.0%), PPMS (50.0%), LGFMS and SEF (50.0%). Although overall FISH testing was more reliable, the additional RT–PCR testing was useful in identifying a fusion transcript containing an EWSR1 rearrangement in FISH-negative cases, particularly for Ewing sarcoma (four cases, 3.6%), DRSCT (two cases, 9.1%), AFH (four cases, 20.0%), CCSLGT (one case, 20.0%) and LGFMS and SEF (six cases, 31.6%).
Unclassifiable EWSR1-rearranged neoplasms
The morphologic and immunohistochemical characteristics of the 13 unclassifiable EWSR1-rearranged neoplasms are summarised in Table 5. This separate group of cancers was more likely to be diagnosed in an older population (median age 55 years, range 11–82 years). There were no identifiable recurrent morphologic characteristics in this group. Despite extensive immunohistochemical studies coupled with morphologic analysis, three cases were classified as spindle cell sarcomas, not otherwise specified, two as possible myoepithelial neoplasms, one as possible EMC, one as possible solitary fibrous tumour, one as possible myxoid spindle cell sarcoma with adipocytic differentiation and the remainder as unclassifiable neoplasms.
Discussion
The two main diagnostic platforms available to routine surgical pathology laboratories are FISH and RT–PCR, which are most beneficial when used as complementary modalities. It is now clear though that the spectrum of gene fusions associated with soft tissue tumours is far wider than previously anticipated. Our study exemplifies the widespread nature of EWSR1 rearrangements in soft tissue tumours and the need to correlate positive molecular findings with morphology and immunohistochemistry. FISH was the more frequently used ancillary test for EWSR1-rearranged neoplasms, often because FISH can be performed with scanty amounts of material (using sections cut at 1–4 μm thickness), compared with RT–PCR which initially required sections cut at 20 μm to isolate sufficient RNA (although this is no longer the case, with 3–5 μm sections now routinely used). Another reason for the prevalence of FISH is the lack of available PCR primers to identify rarer fusion variants. Although RT–PCR still has a higher failure rate than FISH, it is a valuable complementary modality, as exemplified in a minority of cases where a fusion transcript was identified while FISH failed or was negative. Plausible explanations for these results include cases where the percentage of neoplastic cells was low and therefore below the threshold where FISH could assign a positive result (i.e., <15% due to a high immune or stromal component) and the presence of rare translocation structures, where the signal configuration could not be easily interpreted as rearranged. While FISH has traditionally been viewed as the more sensitive test compared with RT–PCR, in some tumours such as AFH, RT–PCR shows an equal sensitivity, and may detect cases that are negative with FISH (Thway et al, 2015b).
There has been a reduction in the technical failure rates of both modalities over time, but notably of RT–PCR. FISH methods improved with the addition of the extra step described in the methods section. The improved success rate in RT–PCR, including with FFPE material from external institutions, is likely multifactorial. The only protocol change over time (which started around 2011) has been the use of 3–5 μm FFPE sections (rather than 20 μm scrolls) and the deparaffinisation on slides rather than in microcentrifuge tubes. In addition, the greater amount of non-microdissected tissue used previously may have saturated PCR columns with paraffin, undigested material or PCR inhibitor carryover (Vroobel et al, 2016). The increasing success in recent years of both RT–PCR and FISH has been markedly aided by the recognition amongst surgical pathology departments of molecular diagnostics (to assess genetic abnormalities in solid neoplasms with available targeted therapies) as a critical component of the patient pathway. This has resulted in widespread improvements from hospitals in fixing and processing pathologic specimens to optimise the tissue available for molecular analysis, with increasing experience of the staff of both surgical pathology and molecular laboratories (Vroobel et al, 2016).
Given the increasing spectrum of EWSR1-rearranged neoplasms, it is clear that the utility and practicality of ancillary molecular tests need re-evaluation, and it is important that physicians are aware of the limitations of molecular diagnostic techniques, including the non-specificity of many gene fusions. The increasing discovery of characteristic genetic abnormalities in soft tissue neoplasms has caused a significant challenge for molecular diagnostics laboratories in maintaining accredited ancillary tests (van de Rijn et al, 2014), compounded by the low cost-benefit ratios due to disease rarity (van de Rijn et al, 2014; Mourtzoukou et al, 2015). It is important not to disregard the value of immunohistochemistry (Hornick, 2014), which is highly cost-effective and widely available in standard surgical pathology laboratories. Immunohistochemical markers that act as surrogates for molecular investigations are likely to be more cost-effective and rapid. For example, Ewing sarcomas with EWSR1-ERG fusions can be detected with the ERG antibody (Wang et al, 2012), although this is not specific, as other neoplasms (including vascular tumours, ERG-related myeloproliferative disorders and prostatic adenocarcinomas) express ERG (Miettinen et al, 2011).
Although helpful in supporting or confirming diagnoses, FISH and RT–PCR continue to present limitations, and this is exemplified by Ewing sarcomas (Figure 1B–D). The t(11;22)(q24;q12) rearrangement leading to EWSR1-FLI1 fusion is the commonest (in ∼85%; Delattre et al, 1992), with about 10% harbouring t(21;22)(q22;q12) leading to EWSR1-ERG fusion (Sorensen et al, 1994; Maire et al, 2008). However, as there are numerous variant fusions (present in less than 1%), ancillary molecular analysis can be negative in tumours displaying typical morphologic and immunohistochemical features of Ewing sarcoma. These variants include the EWSR1-ETS fusions EWSR1-ETV1 (Jeon et al, 1995), EWSR1-ETV4 (Kaneko et al, 1996) and EWSR1-FEV (Peter et al, 1997) and the TET-ETS fusions FUS-ERG (Ichikawa et al, 1994; Berg et al, 2009) and FUS-FEV (Ng et al, 2007; Fisher, 2014), as well as rare non-TET/ETS fusions (Mastrangelo et al, 2000; Yamaguchi et al, 2005; Wang et al, 2007; Szuhai et al, 2009; Sumegi et al, 2011; Fisher, 2014) which are not routinely detected by RT–PCR. Furthermore, a group of primitive round cell tumours with histologic appearances similar to Ewing sarcomas remain unclassifiable, lacking specific clinical and immunohistochemical features and molecular evidence of EWSR1 gene rearrangements or other small round cell tumour-associated gene rearrangements such as SS18, DDIT3 or FOXO1. FUS can act as an alternative to EWSR1 in other neoplasms (Ordonez et al, 2009) although this was not routinely requested in the suspected EWSR1-rearranged neoplasms assessed, as the role and significance of FUS as an alternative to EWSR1 was not as well known in previous years.
The most common genetic abnormality in small round cell tumours lacking EWSR1 rearrangement seems to be the CIC-DUX4 fusion (Italiano et al, 2012), in which CIC on 19q13.2 fuses with one of the DUX4 retrogenes on 4q35 or 10q26.3 (Antonescu, 2014). CIC-DUX4 fusions have been demonstrated in up to two thirds of EWSR1 rearrangement-negative undifferentiated round cell sarcomas of pediatric and young adult patients (Graham et al, 2012; Italiano et al, 2012; Antonescu, 2014), with expression profiling demonstrating a distinct gene signature and suggesting a distinct pathogenesis from Ewing sarcoma (Specht et al, 2014). BCOR-CCNB3-associated round cell neoplasms are also aggressive neoplasms occurring largely in bone or sometimes the deep soft tissues of adolescents or young adults, particularly males (Pierron et al, 2012; Puls et al, 2014). Although there is a range of histologic appearances, most are composed of undifferentiated small round cells similar to Ewing sarcomas. Immunohistochemically they express nuclear CCNB3 (which is thought to be relatively specific), with most also expressing bcl-2 and approximately two thirds positive for CD99 and CD117 (Pierron et al, 2012; Puls et al, 2014). Some of the tumours in the small subset of doubly FISH and RT–PCR negative ‘Ewing sarcomas’ we identified (Table 4) may possibly represent these newly classified entities.
Finally, 13 ‘unclassifiable’ neoplasms which did not show typical histologic or immunohistochemical features of Ewing sarcoma showed EWSR1 rearrangement with FISH but no identifiable partner, that is, they did not harbour detectable EWSR1-related fusion transcripts with the primers available (Table 5) (Figure 2). Histologically these showed a wide spectrum of architectures and cell types, including spindle, round and ovoid cells, and none of these fitted clearly into any particular group of EWSR1-rearranged neoplasms. The new interpretation offered for these was therefore usually of a morphological description such as ‘high grade spindle cell sarcoma.’ These tended to be malignant neoplasms often with cellular atypia, necrosis and mitotic figures. None of these had pathologic features typical of CIC-DUX4- or BCOR-CCNB3-associated sarcomas. It is unclear whether any of these neoplasms could represent specific, as yet uncharacterised tumour types harbouring EWSR1 rearrangements with unknown partner genes, or if these EWSR1 rearrangements might be non-reproducible and a function of the intrinsic genetic instability of the tumours. It seems, however, that ‘unclassifiable’ EWSR1-rearranged neoplasms represent only a small proportion of cases in this study; from these relatively small numbers there were no identifiably recurrent morphologic patterns or immunoprofiles, so the hypothesis of these representing malignant tumours in which EWSR1 rearrangement was present due to inherent genetic instability rather than as a driver mechanism for pathogenesis appears more likely. Recently, investigators have highlighted four cases of SMARCB1-deleted neoplasms of various morphologies (myoepithelial carcinoma, extrarenal rhabdoid tumour, poorly differentiated chordoma and proximal-type epithelioid sarcoma) that also demonstrated EWSR1 abnormalities with FISH, which had initially led to misinterpretation of these as EWSR1-rearranged tumours (Huang et al, 2016). FISH had shown heterozygous deletion or unbalanced split signals for EWSR1, suggesting unbalanced translocations or rearrangements, but these were in keeping with false-positive results owing to the proximity of the SMARCB1 and EWSR1 genes on chromosome 22, whereby large SMARCB1 deletions can involve the EWSR1 locus. Care should therefore be taken in the interpretation of FISH for EWSR1 rearrangement with INI1-deficient neoplasms, and in general the FISH patterns for these tumours have been complex, differing from the more uniform and simple split signals of typical EWSR1-rearranged neoplasms (Huang et al, 2016).
Examples of neoplasms with EWSR1 rearrangement but no corresponding detectable EWSR1 -associated fusion transcripts. (A and B) This is a cellular spindle cell neoplasm on the foot of an adult female. Morphologically, this showed nests and sheets of relatively uniform spindle cells (A) and was immunohistochemically diffusely positive for S100 protein (B). The pathologic features were consistent with clear cell sarcoma (of tendons and aponeuroses), but EWSR1-CREB1 and EWSR1-ATF1 fusion transcripts were undetectable with RT–PCR. This may be due to rarer variant translocations or fusion transcripts that are not detectable with commercial primers. (C and D) This is a neoplasm of sparse to moderate cellularity, composed of patternless distributions of ovoid cells with fibrillary cytoplasm in prominent myxoid stroma with many interspersed thin-walled, medium-sized arcuate vessels. Small numbers of lipoblasts are present (C). While the cells are often bland, focally there is some cytologic atypia (D), and the features were of a tumour with adipocytic differentiation that was not wholly in keeping with myxoid liposarcoma (MLPS). No FUS-DDIT3 fusion transcripts (seen in the majority of MLPS) were detectable with RT–PCR. While FISH showed an EWSR1 rearrangement, no DDIT3 rearrangement was identifiable (which should be present in myxoid liposarcomas with either FUS-DDIT3 or EWSR1-DDIT3 fusions). Therefore this remained an unclassifiable adipocytic neoplasm with myxoid stroma.
Conclusions
This series demonstrates the importance of FISH and RT–PCR as ancillary diagnostic tools in the diagnosis of EWSR1-rearranged neoplasms. Although FISH is more sensitive for identifying EWSR1 rearrangements than RT–PCR, and RT–PCR using commercial primers is limited to only the most prevalent EWSR1 fusion transcripts, their complementary use is crucial as cases in which FISH is negative may demonstrate EWSR1-associated fusion transcripts on RT–PCR. As translocations involving EWSR1 and its partner genes are often not specific for tumour types and there is significant morphologic and immunohistochemical overlap between groups of EWSR1-rearranged neoplasms, it is critical that ancillary molecular findings are always evaluated in specific clinical and pathological context. However, it is also clear that the current routine technologies available in most surgical pathology or molecular diagnostics laboratories (chiefly the two major platforms of FISH and RT–PCR) can only help detect a limited number of fusion genes and these are also low-throughput and labour intensive. The incorporation of high-throughput sequencing technologies into the standard diagnostic repertoire to assess for multiple molecular abnormalities of soft tissue tumours in parallel (including detection of newly characterised Ewing sarcoma-like tumours) might be the most effective and efficient means of ancillary diagnosis in future. This will provide a more definitive classification of many neoplasms that are currently not formally diagnosable with the routine methods available, and enable a specific treatment plan, including entry into appropriate clinical trials for targeted therapies towards specific genetic abnormalities.
References
Antonescu C (2014) Round cell sarcomas beyond Ewing: emerging entities. Histopathology 64: 26–37.
Antonescu C, Tschernyavsky S, Woodruff J, Jungbluth A, Brennan M, Ladanyi M (2002) Molecular diagnosis of clear cell sarcoma: detection of EWS-ATF1 and MITF-M transcripts and histopathological and ultrastructural analysis of 12 cases. J Mol Diagn 4: 44–52.
Antonescu CR, Dal Cin P, Nafa K, Teot LA, Surti U, Fletcher CD, Ladanyi M (2007) EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 46: 1051–1060.
Antonescu C, Zhang L, Chang N-E, Pawel B, Travis W, Katabi N, Edelman M, Rosenberg A, Nielsen GP, Dal Cin P, Fletcher CDM (2010a) EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer 49: 1114–1124.
Antonescu CR, Gerald WL, Magid MS, Ladanyi M (1998) Molecular variants of the EWS-WT1 gene fusion in desmoplastic small round cell tumor. Diagn Mol Pathol 7: 24–28.
Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Katabi N, Edelman M, Rosenberg AE, Nielsen GP, Dal Cin P, Fletcher CD (2010b) EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer 49: 1114–1124.
Arbajian E, Puls F, Magnusson L, Thway K, Fisher C, Sumathi VP, Tayebwa J, Nord KH, Kindblom LG, Mertens F (2014) Recurrent EWSR1-CREB3L1 gene fusions in sclerosing epithelioid fibrosarcoma. Am J Surg Pathol 38: 801–808.
Aurias A, Rimbaut C, Buffe D, Dubousset J, Mazabraud A (1983) [Translocation of chromosome 22 in Ewing's sarcoma]. C R Seances Acad Sci III 296: 1105–1107.
Berg T, Kalsaas AH, Buechner J, Busund LT (2009) Ewing sarcoma-peripheral neuroectodermal tumor of the kidney with a FUS-ERG fusion transcript. Cancer Genet Cytogenet 194: 53–57.
Clark J, Benjamin H, Gill S, Sidhar S, Goodwin G, Crew J, Gusterson BA, Shipley J, Cooper CS (1996) Fusion of the EWS gene to CHN, a member of the steroid/thyroid receptor gene superfamily, in a human myxoid chondrosarcoma. Oncogene 12: 229–235.
Coindre J-M, Hostein I, Terrier P, Bouvier Labit C, Collin F, Michels J-J, Trassard M, Marques B, Ranchere D, Guillou L (2006) Diagnosis of clear cell sarcoma by real-time reverse transcriptase-polymerase chain reaction analysis of paraffin embedded tissues: clinicopathologic and molecular analysis of 44 patients from the French sarcoma group. Cancer 107: 1055–1064.
Dal Cin P, Sciot R, Panagopoulos I, Aman P, Samson I, Mandahl N, Mitelman F, Van den Berghe H, Fletcher CD (1997) Additional evidence of a variant translocation t(12;22) with EWS/CHOP fusion in myxoid liposarcoma: clinicopathological features. J Pathol 182: 437–441.
Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G et al (1992) Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 359: 162–165.
Dockhorn Dworniczak B, Schäfer KL, Dantcheva R, Blasius S, Winkelmann W, Strehl S, Burdach S, van Valen F, Jürgens H, Böcker W (1994) Diagnostic value of the molecular genetic detection of the t(11;22) translocation in Ewing's tumours. Virchows Arch 425: 107–112.
Doyle L, Wang W-L, Dal Cin P, Lopez Terrada D, Mertens F, Lazar AJF, Fletcher CDM, Hornick J (2012) MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol 36: 1444–1451.
Fisher C (2014) The diversity of soft tissue tumours with EWSR1 gene rearrangements: a review. Histopathology 64: 134–150.
Graham C, Chilton-MacNeill S, Zielenska M, Somers GR (2012) The CIC-DUX4 fusion transcript is present in a subgroup of pediatric primitive round cell sarcomas. Hum Pathol 43: 180–189.
Hallor K, Mertens F, Jin Y, Meis Kindblom J, Kindblom L-G, Behrendtz M, Kalén A, Mandahl N, Panagopoulos I (2005) Fusion of the EWSR1 and ATF1 genes without expression of the MITF-M transcript in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 44: 97–102.
Hisaoka M, Ishida T, Kuo T-T, Matsuyama A, Imamura T, Nishida K, Kuroda H, Inayama Y, Oshiro H, Kobayashi H, Nakajima T, Fukuda T, Ae K, Hashimoto H (2008) Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol 32: 452–460.
Hornick JL (2014) Novel uses of immunohistochemistry in the diagnosis and classification of soft tissue tumors. Mod Pathol 27 (Suppl 1): S47–S63.
Hosaka T, Nakashima Y, Kusuzaki K, Murata H, Nakayama T, Nakamata T, Aoyama T, Okamoto T, Nishijo K, Araki N, Tsuboyama T, Nakamura T, Toguchida J (2002) A novel type of EWS-CHOP fusion gene in two cases of myxoid liposarcoma. J Mol Diagn 4: 164–171.
Hostein I, Andraud Fregeville M, Guillou L, Terrier Lacombe M-J, Deminière C, Ranchère D, Lussan C, Longavenne E, Bui N, Delattre O, Coindre J-M (2004) Rhabdomyosarcoma: value of myogenin expression analysis and molecular testing in diagnosing the alveolar subtype: an analysis of 109 paraffin-embedded specimens. Cancer 101: 2817–2824.
Huang SC, Zhang L, Sung YS, Chen CL, Kao YC, Agaram NP, Antonescu CR (2016) Secondary EWSR1 gene abnormalities in SMARCB1-deficient tumors with 22q11-12 regional deletions: Potential pitfalls in interpreting EWSR1 FISH results. Genes Chromosomes Cancer 55: 767–776.
Ichikawa H, Shimizu K, Hayashi Y, Ohki M (1994) An RNA-binding protein gene, TLS/FUS, is fused to ERG in human myeloid leukemia with t(16;21) chromosomal translocation. Cancer Res 54: 2865–2868.
Italiano A, Sung YS, Zhang L, Singer S, Maki RG, Coindre JM, Antonescu CR (2012) High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosomes Cancer 51: 207–218.
Jeon IS, Davis JN, Braun BS, Sublett JE, Roussel MF, Denny CT, Shapiro DN (1995) A variant Ewing's sarcoma translocation (7;22) fuses the EWS gene to the ETS gene ETV1. Oncogene 10: 1229–1234.
Jin L, Majerus J, Oliveira A, Inwards CY, Nascimento AG, Burgart LJ, Lloyd RV (2003) Detection of fusion gene transcripts in fresh-frozen and formalin-fixed paraffin-embedded tissue sections of soft-tissue sarcomas after laser capture microdissection and rt-PCR. Diagn Mol Pathol 12: 224–230.
Kaneko Y, Yoshida K, Handa M, Toyoda Y, Nishihira H, Tanaka Y, Sasaki Y, Ishida S, Higashino F, Fujinaga K (1996) Fusion of an ETS-family gene, EIAF, to EWS by t(17;22)(q12;q12) chromosome translocation in an undifferentiated sarcoma of infancy. Genes Chromosomes Cancer 15: 115–121.
Ladanyi M, Gerald W (1994) Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res 54: 2837–2840.
Lau PPL, Lui PCW, Yau DTW, Cheung ETY, Chan JKC (2013) EWSR1-CREB3L1 gene fusion: a novel alternative molecular aberration of low-grade fibromyxoid sarcoma. Am J Surg Pathol 37: 734–738.
Lewis T, Coffin C, Bernard P (2007) Differentiating Ewing's sarcoma from other round blue cell tumors using a RT-PCR translocation panel on formalin-fixed paraffin-embedded tissues. Mod Pathol 20: 397–404.
Li H, Watford W, Li C, Parmelee A, Bryant M, Deng C, O'Shea J, Lee S (2007) Ewing sarcoma gene EWS is essential for meiosis and B lymphocyte development. J Clin Invest 117: 1314–1323.
Maire G, Brown CW, Bayani J, Pereira C, Gravel DH, Bell JC, Zielenska M, Squire JA (2008) Complex rearrangement of chromosomes 19, 21, and 22 in Ewing sarcoma involving a novel reciprocal inversion-insertion mechanism of EWS-ERG fusion gene formation: a case analysis and literature review. Cancer Genet Cytogenet 181: 81–92.
Mastrangelo T, Modena P, Tornielli S, Bullrich F, Testi MA, Mezzelani A, Radice P, Azzarelli A, Pilotti S, Croce CM, Pierotti MA, Sozzi G (2000) A novel zinc finger gene is fused to EWS in small round cell tumor. Oncogene 19: 3799–3804.
Mertens F, Tayebwa J (2014) Evolving techniques for gene fusion detection in soft tissue tumours. Histopathology 64: 151–162.
Miettinen M, Wang ZF, Paetau A, Tan SH, Dobi A, Srivastava S, Sesterhenn I (2011) ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol 35: 432–441.
Mourtzoukou D, Fisher C, Thway K (2015) Evaluation of molecular and immunohistochemical adjunct modalities in the diagnosis of soft tissue neoplasms. Int J Surg Pathol 23: 601–608.
Ng TL, O'Sullivan MJ, Pallen CJ, Hayes M, Clarkson PW, Winstanley M, Sorensen PH, Nielsen TO, Horsman DE (2007) Ewing sarcoma with novel translocation t(2;16) producing an in-frame fusion of FUS and FEV. J Mol Diagn 9: 459–463.
Ordonez JL, Osuna D, Herrero D, de Alava E, Madoz-Gurpide J (2009) Advances in Ewing’s sarcoma research: where are we now and what lies ahead? Cancer Res 69: 7140–7150.
Okamoto S, Hisaoka M, Ishida T, Imamura T, Kanda H, Shimajiri S, Hashimoto H (2001) Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and molecular analysis of 18 cases. Hum Pathol 32: 1116–1124.
Panagopoulos I, Hoglund M, Mertens F, Mandahl N, Mitelman F, Aman P (1996) Fusion of the EWS and CHOP genes in myxoid liposarcoma. Oncogene 12: 489–494.
Paronetto M (2013) Ewing sarcoma protein: a key player in human cancer. Int J Cell Biol 2013: 642853.
Peter M, Couturier J, Pacquement H, Michon J, Thomas G, Magdelenat H, Delattre O (1997) A new member of the ETS family fused to EWS in Ewing tumors. Oncogene 14: 1159–1164.
Pierron G, Tirode F, Lucchesi C, Reynaud S, Ballet S, Cohen-Gogo S, Perrin V, Coindre JM, Delattre O (2012) A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet 44: 461–466.
Puls F, Niblett A, Marland G, Gaston CL, Douis H, Mangham DC, Sumathi VP, Kindblom LG (2014) BCOR-CCNB3 (Ewing-like) sarcoma: a clinicopathologic analysis of 10 cases, in comparison with conventional Ewing sarcoma. Am J Surg Pathol 38: 1307–1318.
Rossi S, Szuhai K, Ijszenga M, Tanke H, Zanatta L, Sciot R, Fletcher CDM, Dei Tos A, Hogendoorn PCW (2007) EWSR1-CREB1 and EWSR1-ATF1 fusion genes in angiomatoid fibrous histiocytoma. Clin Cancer Res 13: 7322–7328.
Sciot R, Dal Cin P, Fletcher C, Samson I, Smith M, De Vos R, Van Damme B, Van den Berghe H (1995) t(9;22)(q22-31;q11-12) is a consistent marker of extraskeletal myxoid chondrosarcoma: evaluation of three cases. Mod Pathol 8: 765–768.
Sorensen PH, Lessnick SL, Lopez-Terrada D, Liu XF, Triche TJ, Denny CT (1994) A second Ewing's sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat Genet 6: 146–151.
Specht K, Sung YS, Zhang L, Richter GH, Fletcher CD, Antonescu CR (2014) Distinct transcriptional signature and immunoprofile of CIC-DUX4 fusion-positive round cell tumors compared to EWSR1-rearranged Ewing sarcomas: further evidence toward distinct pathologic entities. Genes Chromosomes Cancer 53: 622–633.
Sumegi J, Nishio J, Nelson M, Frayer RW, Perry D, Bridge JA (2011) A novel t(4;22)(q31;q12) produces an EWSR1-SMARCA5 fusion in extraskeletal Ewing sarcoma/primitive neuroectodermal tumor. Mod Pathol 24: 333–342.
Szuhai K, Ijszenga M, de Jong D, Karseladze A, Tanke HJ, Hogendoorn PC (2009) The NFATc2 gene is involved in a novel cloned translocation in a Ewing sarcoma variant that couples its function in immunology to oncology. Clin Cancer Res 15: 2259–2268.
Thway K, Bown N, Miah A, Turner R, Fisher C (2015a) Rhabdoid variant of myoepithelial carcinoma, with EWSR1 rearrangement: expanding the spectrum of EWSR1-rearranged myoepithelial tumors. Head Neck Pathol 9: 273–279.
Thway K, Fisher C (2012) Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol 36: e1–e11.
Thway K, Fisher C (2014) Myoepithelial tumor of soft tissue: histology and genetics of an evolving entity. Adv Anat Pathol 21: 411–419.
Thway K, Fisher C (2015) Angiomatoid fibrous histiocytoma: the current status of pathology and genetics. Arch Pathol Lab Med 139: 674–682.
Thway K, Gonzalez D, Wren D, Dainton M, Swansbury J, Fisher C (2015b) Angiomatoid fibrous histiocytoma: comparison of fluorescence in situ hybridization and reverse transcription polymerase chain reaction as adjunct diagnostic modalities. Ann Diagn Pathol 19: 137–142.
Thway K, Nicholson A, Lawson K, Gonzalez D, Rice A, Balzer B, Swansbury J, Min T, Thompson L, Adu Poku K, Campbell A, Fisher C (2011) Primary pulmonary myxoid sarcoma with EWSR1-CREB1 fusion: a new tumor entity. Am J Surg Pathol 35: 1722–1732.
Thway K, Wren D, Lee J, Thompson L, Fisher C, Gonzalez D (2016) Evaluation of the optimal provision of formalin-fixed, paraffin-embedded material for reverse transcription-PCR in soft-tissue tumour diagnosis. J Clin Pathol 70: 20–24.
van de Rijn M, Guo X, Sweeney RT, Beck AH, West RB (2014) Molecular pathological analysis of sarcomas using paraffin-embedded tissue: current limitations and future possibilities. Histopathology 64: 163–170.
Vroobel K, Gonzalez D, Wren D, Thompson L, Swansbury J, Fisher C, Thway K (2016) Ancillary molecular analysis in the diagnosis of soft tissue tumours: reassessment of its utility at a specialist centre. J Clin Pathol 69: 505–510.
Wang J, Thway K (2015) Clear cell sarcoma-like tumor of the gastrointestinal tract: an evolving entity. Arch Pathol Lab Med 139: 407–412.
Wang L, Bhargava R, Zheng T, Wexler L, Collins MH, Roulston D, Ladanyi M (2007) Undifferentiated small round cell sarcomas with rare EWS gene fusions: identification of a novel EWS-SP3 fusion and of additional cases with the EWS-ETV1 and EWS-FEV fusions. J Mol Diagn 9: 498–509.
Wang W-L, Mayordomo E, Zhang W, Hernandez V, Tuvin D, Garcia L, Lev D, Lazar A, López Terrada D (2009) Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts). Mod Pathol 22: 1201–1209.
Wang WL, Patel NR, Caragea M, Hogendoorn PC, Lopez-Terrada D, Hornick JL, Lazar AJ (2012) Expression of ERG, an Ets family transcription factor, identifies ERG-rearranged Ewing sarcoma. Mod Pathol 25: 1378–1383.
Yamaguchi S, Yamazaki Y, Ishikawa Y, Kawaguchi N, Mukai H, Nakamura T (2005) EWSR1 is fused to POU5F1 in a bone tumor with translocation t(6;22)(p21;q12). Genes Chromosomes Cancer 43: 217–222.
Acknowledgements
We acknowledge support from the NIHR Royal Marsden/ICR Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Noujaim, J., Jones, R., Swansbury, J. et al. The spectrum of EWSR1-rearranged neoplasms at a tertiary sarcoma centre; assessing 772 tumour specimens and the value of current ancillary molecular diagnostic modalities. Br J Cancer 116, 669–678 (2017). https://doi.org/10.1038/bjc.2017.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.4
Keywords
This article is cited by
-
Molecular mechanisms underpinning sarcomas and implications for current and future therapy
Signal Transduction and Targeted Therapy (2021)
-
The epigenomics of sarcoma
Nature Reviews Cancer (2020)
-
A novel next generation sequencing approach to improve sarcoma diagnosis
Modern Pathology (2020)
-
Primary paediatric epidural sarcomas: molecular exploration of three cases
BMC Cancer (2019)
-
Limited biopsies of soft tissue tumors: the contemporary role of immunohistochemistry and molecular diagnostics
Modern Pathology (2019)