Abstract
Background:
Several studies have identified L1 cell adhesion molecule (L1CAM) as a strong prognostic marker in endometrial cancer. To further underline the clinical usefulness of this biomarker, we investigated L1CAM as a predictive marker for lymph node metastases and its prognostic impact in curettage specimens and preoperative plasma samples. In addition, we aimed to validate the prognostic value of L1CAM in hysterectomy specimen.
Methods:
Immunohistochemical staining of L1CAM was performed for 795 hysterectomy and 1134 curettage specimen from endometrial cancer patients. The L1CAM level in preoperative blood samples from 372 patients was determined using ELISA.
Results:
Expression of L1CAM in curettage specimen was significantly correlated to L1CAM level in corresponding hysterectomy specimen (P<0.001). Both in curettage and preoperative plasma samples L1CAM upregulation was significantly associated with features of aggressive disease and poor outcome (P<0.001). The L1CAM was an independent predictor of lymph node metastases, after correction for curettage histology, both in curettage specimen (P=0.002) and plasma samples (P=0.048). In the hysterectomy samples L1CAM was significantly associated with poor outcome (P<0.001).
Conclusions:
We demonstrate that preoperative evaluation of L1CAM levels, both in curettage or plasma samples, predicts lymph node metastases and adds valuable information on patient prognosis.
Similar content being viewed by others
Main
Endometrial cancer is the most common gynaecologic malignancy and the fourth most common cancer among women in industrialised countries (Torre et al, 2015). Although the prognosis is good, 15 to 20% of patients with presumed localised disease at diagnosis recur (Abeler and Kjorstad, 1991; Morrow et al, 1991). Identifying biomarkers that can select patient populations for optimal surgical and systemic treatment is important to improve outcome for these patients.
The diagnosis and treatment of endometrial cancer patients usually consists of a preoperative biopsy followed by imaging, surgery and adjuvant treatment depending on risk classification. A complete surgical staging of endometrial cancer patients involves both pelvic and para-aortic lymphadenectomy (Pecorelli, 2009). Worldwide, there are however a wide variation in lymphadenectomy in endometrial cancer, and selection criteria for lymphadenectomy are not uniformly standardised (Maggino et al, 1995, 1998). Metastases to the lymph nodes are known to be associated with poor prognosis, and although lymphadenectomy provides diagnostic information that can help in selecting optimal adjuvant treatment, its effect on survival is uncertain, and it is associated with increased complication rates (Benedetti Panici et al, 2008; Wright et al, 2012; Morice et al, 2016). Improved identification of high-risk patients preoperatively is therefore needed to tailor the primary surgical strategy.
L1 cell adhesion molecule (L1CAM) is a cell adhesion molecule of the immunoglobulin superfamily (Moos et al, 1988). It consists of an extracellular domain, a transmembrane region and a highly conserved cytoplasmic domain (Moos et al, 1988). The extracellular domain of L1CAM can be cleaved by the metalloproteases ADAM10 and ADAM17, resulting in a soluble form of L1CAM (Mechtersheimer et al, 2001; Maretzky et al, 2005). The L1CAM was initially identified in the nervous system and plays an important role in neurogenesis (Rathjen and Schachner, 1984; Moos et al, 1988; Schafer and Altevogt, 2010). Later, L1CAM has also been shown to be involved in almost every aspect of cancer progression including promoting cell proliferation, migration, invasion and metastases of cancer cells (Raveh et al, 2009; Schafer and Altevogt, 2010; Altevogt et al, 2016).
Expression of L1CAM has been investigated in several cancer types, and is associated with poor outcome (Fogel et al, 2003; Allory et al, 2005; Boo et al, 2007; Schroder et al, 2009; Tischler et al, 2011; Tsutsumi et al, 2011; Wang et al, 2013; Hua et al, 2016). In endometrial cancer hysterectomy samples, L1CAM has been reported to be associated with aggressive disease characteristics including loss of hormone receptors and reduced survival (Huszar et al, 2010; Zeimet et al, 2013; Bosse et al, 2014; Dellinger et al, 2016; Geels et al, 2016; van der Putten et al, 2016; Kommoss et al, 2017). The L1CAM is a strong prognostic marker within the subgroup of stage I endometrioid endometrial cancer (Zeimet et al, 2013; van der Putten et al, 2016), and within the subgroup of advanced-stage endometrioid endometrial cancer (van der Putten et al, 2016), when evaluated postoperatively in hysterectomy samples. However, the expression of L1CAM in preoperative biopsies and its usefulness in preoperative prediction of lymph node metastases and outcome are not yet established. In addition, soluble L1CAM (sL1CAM) is suggested to be a valuable circulating biomarker in different cancers (Zander et al, 2011; Bondong et al, 2012). It has been detected in the culture medium from different cell lines including melanoma, breast and ovarian cancer (Beer et al, 1999; Gutwein et al, 2005; Li and Galileo, 2010), and also in serum and ascites of endometrial and ovarian cancer (Fogel et al, 2003; Wojciechowski et al, 2017), suggesting that the soluble form of L1CAM may also play a role in these cancers.
The primary objective of this study was to assess the value of L1CAM in preoperative samples (curettage and plasma samples) as a predictive marker for lymph node metastases and poor prognosis. In addition, we aimed to validate the prognostic value of L1CAM in hysterectomy samples.
Materials and methods
Patient series
A population-based series was prospectively collected from 2001 to 2014 and included curettage specimen (n=600), primary tumours (n=795, of which 189 overlapped with cases published by van der Putten et al (van der Putten et al, 2016)) and plasma (n=372) from patients diagnosed with endometrial cancer in Hordaland County (Norway). Within the MoMaTEC study (Molecular markers in treatment of Endometrial Cancer, NCT00598845), nine other centres contributed with preoperative biopsies from in total 534 patients treated for endometrial cancer prospectively included at their institutions. Formalin-fixed, paraffin-embedded (FFPE) tumour tissue from curettage specimen was collected from all participating institutions (Trovik et al, 2013). Clinical and histopathological data were collected, including age, FIGO stage (according to 2009 criteria), histologic type and grade and myometrial invasion from hysterectomy specimen. Follow-up data were collected as previously described (Trovik et al, 2011). Preoperative histology reports were categorised as high risk (serous, clear cell, carcinosarcoma, undifferentiated carcinomas, endometrioid grade 3) or low risk (benign (diagnosed with endometrial cancer after hysterectomy), hyperplasia, endometrioid grades 1–2). Pelvic lymphadenectomy as part of surgical staging was conducted in the majority of cases (n=825, 73%); of these, additional para-aortic lymph node sampling was conducted for 6% (n=49). Fresh frozen tissue from hysterectomy samples was investigated for L1CAM mRNA expression in 232 patients, and these overlapped with FFPE samples.
The study has been approved by Norwegian legislation, including the Norwegian Data Inspectorate, Norwegian Social Science Data Services and the Western Regional Committee for Medical and Health Research Ethics (REK 2009/2315). All patients signed informed consent before inclusion in the study.
Immunohistochemical staining
The FFPE tissue from curettage and hysterectomy samples was available in tissue microarrays (TMAs) from 1134 and 795 patients, respectively, for evaluation of L1CAM level. The TMAs were generated from FFPE tissue as previously described, with three tissue cylinders from each case (Stefansson et al, 2004). The TMAs were dewaxed in xylene and rehydrated in graded ethanol series before microwave boiling in target retrieval buffer (pH 9) for 15 min. The L1CAM was detected using purified anti-CD171 (L1) antibody clone 14.10 (Biolegend, San Diego, CA, USA) diluted 1 : 100 for 60 min at room temperature, followed by 30 min of incubation with secondary HPR-conjugated antibody. Diaminobenzidine was applied for 8 min before counterstaining with haematoxylin. All slides were scored independently and blinded for clinical and pathological data by two authors using standard light microscopy. Inter-evaluator κ-value was calculated to be 0.76 for L1CAM in two groups. The staining was evaluated as previously described (Engelsen et al, 2008). Briefly, both intensity and area of positive tumour cells were considered. The intensity of staining was graded from 0 (no staining) to 3 (strong), and the area as 0, 1 (<10%), 2 (10–50%) and 3 (51–100%). From this, a staining index (0–9) was calculated as the product of intensity and area. If heterogeneity was seen for the three tissue cylinders of each case, the three cylinders were given one overall averaged score. In subsequent statistical analysis indexes were grouped according to similarity in survival and considering the size of the subgroup and the number of events in each category. For L1CAM index 0–3 was considered low and 4–9 high (Supplementary Figure 1). Immunohistochemical (IHC) staining and staining evaluation of ER and PR has previously been described (Trovik et al, 2013). Using the same scoring system as above, ER staining index ⩽3 and PR staining index 0 was defined as low expression/loss.
Enzyme-linked immunosorbent assay (ELISA)
The EDTA-blood was obtained from 372 patients with endometrial cancer before primary surgery. The blood samples were centrifuged at 1600 g for 15 min and the plasma was stored at −80 °C until measurement of sL1CAM. The ELISA kits were bought from MyBioSource (Cat. No. MBS2023094, MyBioSource, San Diego, CA, USA) and the sandwich ELISA was performed according to the manufacturer’s instructions. Briefly, 100 μl plasma sample or standard was put into a 96-well microplate and incubated for 2 h at 37 °C. Then, 100 μl biotin-conjugated L1CAM-specific antibody was added and incubated for 1 h at 37 °C. After the well was washed three times with washing solution, 100 μl avidin conjugated to horseradish peroxidase (HRP) was added and incubated for 30 min at 37 °C. The wells were washed five times and 90 μl substrate solution was added before 20 min of incubation away from light followed by addition of 50 μl stop solution. The absorbance was measured in a microplate reader at the wavelength of 450 nm, and plasma concentration of sL1CAM calculated. For further statistical analysis the patients were grouped in four groups based on sL1CAM plasma level, and subsequently in high and low L1CAM based on similarities in survival (the three groups with lowest L1CAM level=low L1CAM level; the upper quartile=high L1CAM level) (Supplementary Figure 1). Plasma from healthy age-matched female blood donors (n=32) was used as control group.
Gene expression analysis
The RNA was extracted from fresh frozen tissue from primary tumour from patients diagnosed with endometrial cancer. Hybridisation to Agilent (Santa Clara, CA, USA) Whole Human Genome Microarray 44k (Cat. No. G4112F) was done according to the manufacturer’s instructions. The arrays were scanned and normalised as previously described (Krakstad et al, 2012).
Statistical analyses
Data were analysed using SPSS version 24 (SPSS Inc., Chicago, IL, USA). Probability of <0.05 was considered statistical significant, and all statistical tests were two sided. Associations between groups were analysed using the χ2 test for categorical variable and the Mann–Whitney U-test for continuous variables. Binary logistic regression was used to estimate odds ratios (ORs) for lymph node metastases. Univariate survival analysis was performed using the Kaplan–Meier (product-limit) method. Disease-specific survival was defined as time from surgery to death from endometrial cancer. Survival between groups was compared using the log-rank (Mantel–Cox) test. The Cox proportional hazard regression model was used to evaluate the prognostic impact of L1CAM adjusted for other prognostic parameters.
Results
Expression of L1CAM in endometrial cancer curettage predicts lymph node metastases and poor outcome
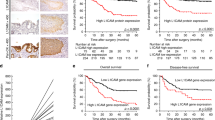
The protein expression of L1CAM was evaluated by IHC in curettage samples from 1134 patients. Low expression was defined as staining index 0–3 and was found in 88% (n=1000) of the lesions, whereas 12% (n=134) expressed high levels of L1CAM. High L1CAM expression in curettage specimen was significantly associated with high age, loss of ER and PR expression and high-risk curettage classification and also high FIGO stage, non-endometrioid histology and high grade in the hysterectomy specimen (all P<0.001) (Table 1). There was a highly significant correlation between L1CAM staining in curettage specimen and staining in the corresponding hysterectomy specimen (P<0.001) (Table 1). High expression of L1CAM in curettage specimen predicted poor disease-specific survival, both in the whole patient population (P<0.001) (Figure 1A) and within patients with low-risk curettage histology (P<0.001) (Figure 1B). Expression of L1CAM also showed independent prognostic impact in Cox survival analysis after correction for age, FIGO stage, histologic subtype and grade assessed in the hysterectomy specimens, with hazard ratio of 1.77 (95% CI 1.17–2.66, P=0.006) (Supplementary Table 1).
Expression of L1CAM is a prognostic marker in curettage samples, blood samples and hysterectomy samples of endometrial cancer patients. High expression of L1CAM in curettage samples was significantly associated with poor outcome in the whole patient population (A) and in the subgroup of patients with low-risk histology in curettage (B). In plasma from endometrial cancer patients, high level of L1CAM was predictive of poor outcome in both the whole patient population (C) and the subgroup of patients with low-risk histology in curettage (D). High expression of L1CAM in hysterectomy samples is significantly associated with poor survival in both the whole population (E) and the subgroup with stage 1 endometrioid endometrial cancer (F). *Curettage histological risk classification, low risk (benign, hyperplasia or endometrioid grades 1–2) or high risk (non-endometrioid or endometrioid grade 3). Number in brackets: number of patients in the group/number of events in the group.
Interestingly, patients with high L1CAM expression in curettage specimen had significantly higher occurrence of lymph node metastases compared with patients with low expression of L1CAM, both in the whole patient population (33% vs 10% respectively, P<0.001, Table 1) and in the subgroup with low-risk histology classification (30% vs 9% respectively, P<0.001, Supplementary Table 2). In a univariate model, curettage high-risk histology, combined loss of ER/PR (a marker previously shown to be a strong predictor of lymph node metastases in endometrial cancer patients; Trovik et al, 2013) and high expression of L1CAM all predicted presence of metastatic lymph nodes (Table 2). When adjusting for curettage histology and preoperative ER/PR loss in a multivariate model, high expression of L1CAM predicted lymph node metastases with adjusted OR 2.51 (95% CI 1.41–4.64, P=0.002) (Table 2).
High sL1CAM level in preoperative blood samples is associated with lymph node metastases and poor survival in endometrial cancer patients
The level of sL1CAM was also investigated in preoperative plasma samples from 372 patients with endometrial cancer. The plasma level of sL1CAM was significantly higher in endometrial cancer patients compared with healthy controls (P=0.001 (endometrial cancer patients: mean=997 pg ml−1, s.e.m.=27; healthy controls: mean=684 pg ml−1, s.e.m.=29)). There was a significant correlation between sL1CAM level in plasma evaluated by ELISA and L1CAM expression in both curettage specimen (P=0.007) (Supplementary Figure 2A) and hysterectomy specimen (P=0.015) (Supplementary Figure 2B) evaluated by IHC. High preoperative plasma levels of sL1CAM were significantly associated with high age, loss of ER and PR and high-risk histology in curettage, and also high FIGO stage and non-endometrioid histology in the hysterectomy specimen (Table 3). High sL1CAM plasma level predicted poor disease-specific survival in both the whole population (P<0.001) (Figure 1C) and the group with low-risk curettage histology (P=0.04) (Figure 1D). sL1CAM level in plasma did not have independent prognostic impact when adjusting for age, FIGO stage, histologic type and grade (data not shown).
Patients with high sL1CAM plasma levels had significantly higher occurrence of lymph node metastases compared with patients with low sL1CAM level (23% vs 9% respectively, P=0.003) when including the whole patent population, but not within the subgroup with low-risk histology classification (Supplementary Table 2). In a univariate model both high-risk histology in curettage and high plasma levels of sL1CAM were predictive of lymph node metastases. In addition, when correcting for curettage histology in a multivariate model, sL1CAM level in plasma was a predictor of lymph node metastases with OR 2.25 (95% CI 1.01–5.02, P=0.048) (Table 4).
Expression of L1CAM in hysterectomy specimen validates to be a strong predictor of poor outcome in endometrial cancer
Several studies have identified L1CAM as a strong prognostic marker when examining hysterectomy specimens in endometrial cancer. In this study we investigated expression of L1CAM in 795 primary tumours. In 14% of the cases (n=110), expression of L1CAM was high whereas 86% (n=685) of the cases showed low L1CAM levels. There was a significant association between L1CAM protein levels and L1CAM mRNA levels (Supplementary Figure 3). We identified that high expression of L1CAM in hysterectomy samples is associated with features of aggressive endometrial cancer (Supplementary Table 3) and poor survival (Figure 1E). Expression of L1CAM also showed independent prognostic impact in Cox survival analysis when adjusting for age, FIGO stage, histologic subtype and grade with HR 2.7 (95% CI 1.8–4.3, P<0.001) (Supplementary Table 4). Within the subgroup of stage I endometrioid endometrial cancer, 5% of the cases expressed high L1CAM. In addition, within this subgroup, expression of L1CAM was associated with characteristics of aggressive endometrial cancer (Supplementary Table 5) and poor survival (Figure 1F), but it did not have independent prognostic impact when adjusting for age and histologic grade (data not shown).
Discussion
There are several studies suggesting L1CAM as a promising biomarker in endometrial cancer, and its expression has earlier been shown to be associated with aggressive disease and poor survival, and inversely correlated with expression of ER and PR (Huszar et al, 2010; Zeimet et al, 2013; Bosse et al, 2014; Dellinger et al, 2016; Geels et al, 2016; van der Putten et al, 2016; Kommoss et al, 2017). Current data point to an added prognostic value of L1CAM, and integrating L1CAM status as part of the molecular classification of endometrial cancer can be useful. We here validate that L1CAM is a strong prognostic marker in hysterectomy samples when including all samples in this prospectively collected population-based series. In addition, within the subgroup of patients with FIGO stage 1 endometrioid endometrial cancer, L1CAM expression is a predictor of poor survival, although not as strong as shown in other studies (Zeimet et al, 2013; van der Putten et al, 2016). The usefulness of L1CAM as a prognostic marker within this subgroup was also questioned in a recent study including 388 patients where L1CAM failed to be a clinically relevant marker of poor prognosis in FIGO stage 1 endometrioid endometrial cancer (Smogeli et al, 2016). These differences could be explained by not only different scoring methods and cutoffs for L1CAM, but also differences in the patient cohort with regard to proportion of patients where lymphadenectomy is performed, as well as the use of adjuvant treatment.
In this study TMAs were used to determine the expression of L1CAM, and the staining index was used as scoring method with high expression defined as 4–9 and low expression defined as 0–3. Most other studies investigating the expression of L1CAM have used whole sections and a cutoff point of 10% (Zeimet et al, 2013; Bosse et al, 2014; Smogeli et al, 2016; van der Putten et al, 2016). The use of different scoring methods and cutoffs make the studies less comparable; however, the fact that different scoring methods identify L1CAM as a strong biomarker supports its robustness as biomarker in endometrial cancer. Although an interobserver κ-value of 0.76 was obtained for L1CAM in this study, a scoring method like the staining index where both intensity and area are taken into account may be more subjectively influenced compared with the method only considering the area. Before potential clinical implementation of L1CAM as biomarker in endometrial cancer, the optimal staining protocol, scoring method and cutoff will have to be determined and validated. In an investigational setting the use of TMAs is both time and cost effective, and the method has been shown to yield reproducible results compared with full sections (Kononen et al, 1998; Fons et al, 2007). However, TMAs do not provide the same morphological information as full sections, and should be used with caution, and depending on the research question. Although three tissue cores were used for each patient in this study, L1CAM-positive areas of the tumour might be missed that may result in underestimation of L1CAM expression. This is however also a challenge using full sections, and validation of markers is crucial before applied in a clinical setting.
In endometrial cancer the tumour is easily accessible for biopsy before surgery. Histologic type and grade are routinely investigated in the preoperative biopsies, and this provides prognostic information relevant for the extent of the surgery. However, the correlation between preoperative assessment of curettage and postoperative evaluation of hysterectomy specimens varies (Lampe et al, 1995; Frumovitz et al, 2004; Eltabbakh et al, 2005; Leitao et al, 2008; Werner et al, 2013). Therefore, identification of reliable markers in curettage material that could predict lymph node metastases would provide a better basis for treatment selection. There are different molecular markers that have been studied in preoperative biopsies, and could serve as predictive markers for metastatic disease (Salvesen et al, 2012). One example is combined loss of ER and PR for prediction of lymph node metastases and poor survival in endometrial cancer (Trovik et al, 2013). Its usefulness in tailoring surgical treatment is currently tested in a clinical trial (Momatec2, NCT02543710), where the decision to perform lymphadenectomy in low- and intermediate-risk patients is dependent on the preoperative hormone receptor status. In the present study we investigate L1CAM level in curettage and preoperative blood samples, and its potential for predicting lymph node metastases and poor prognosis. Only few studies have previously investigated the expression of L1CAM in curettage samples. Bosse et al (2014) compared expression of L1CAM in curettage and hysterectomy samples from 42 patients and Fogel et al (2003) from 14 patients. Both studies reported a good concordance between staining in curettage and hysterectomy samples (Fogel et al, 2003; Bosse et al, 2014). We here confirm that the L1CAM staining in the curettage and hysterectomy sample is significantly associated. More importantly, we report the novel finding that high L1CAM expression in curettage samples is a significant predictor of lymph node metastases in both a univariate analysis and a multivariate analysis correcting for curettage histology and preoperative ER/PR expression. Our large study shows that L1CAM expression in curettage specimens is associated with features of aggressive endometrial cancer disease and poor survival in both the whole patient population and within the subgroup of patients with a preoperative low-risk histology classification. These findings suggest that evaluation of L1CAM in curettage samples could be a valuable supplement to the currently performed preoperative assessment for determination of surgical extent. However, this is the first study investigating L1CAM as a predictive marker for lymph node metastases and its prognostic impact in curettage specimens, and these findings would have to be validated in other studies before its place in the preoperative assessment can be determined.
The soluble form of L1CAM has earlier shown to be present in sera of endometrial cancer patients. In a study by Fogel et al (2003), 9 out of 10 patients with L1CAM-positive endometrial tumours also had detectable concentrations of sL1CAM in preoperative serum samples. A recent study investigating serum levels of sL1CAM in 35 endometrial cancer patients found it to be lower in patients compared with healthy controls, and no correlations between soluble L1CAM concentration and histopathology, stage or grade were found (Wojciechowski et al, 2017). The latter is contradictory to our results as we find that the level of sL1CAM is significantly increased in endometrial cancer patients compared with healthy controls, and that a high level of sL1CAM is associated with aggressive disease characteristics and poor survival. In addition, we report that a high level of sL1CAM in preoperative blood samples predicts lymph node metastasis in both a univariate analysis and a multivariate analysis correcting for preoperative histology. Increased level of sL1CAM has also been found to be associated with poor prognosis in other cancer types, such as in gastrointestinal stromal tumours (Zander et al, 2011) and ovarian cancer (Bondong et al, 2012). Whether the soluble form of L1CAM, in addition to serving as a prognostic biomarker, also has a role in tumour development and progression is not clear, but studies investigating its function have suggested that sL1CAM is involved in stimulating cell motility and contributes to cell survival through activating anti-apoptotic pathways (Mechtersheimer et al, 2001; Bondong et al, 2012).
The primary treatment for endometrial cancer patients is surgical that typically includes hysterectomy and bilateral salpingo-oophorectomy with or without lymphadenectomy. Although lymphadenectomy is part of the complete staging procedure and important for risk stratification of endometrial cancer patients, no survival benefit is shown in randomised clinical trials, and it is associated with increased complication rates and prolonged operation time in a comorbid and obese patient population (Benedetti Panici et al, 2008; Pecorelli, 2009; Wright et al, 2012; Morice et al, 2016). Identifying markers that can preoperatively predict prognosis and lymph node metastases is important. Biomarkers can aid in tailoring the surgical treatment through identifying those patients with advanced disease who would benefit from extensive surgery, but also aid in preventing overtreatment in low-risk patients. Both L1CAM expression evaluated by IHC in curettage and the level of sL1CAM evaluated by ELISA in plasma seem to be promising biomarkers in endometrial cancer. Although L1CAM expression in curettage is a stronger predictor of both lymph node metastases and disease-specific survival in multivariate analysis compared with sL1CAM in plasma, and may be the preferred method, the fact that no tissue and only a blood sample is necessary for the sL1CAM analysis is an advantage. However, additional studies and in particular prospective randomised trials would be important to evaluate the effect of implementing L1CAM expression in curettage samples and sL1CAM level in blood samples into routine clinical practice.
Change history
05 September 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abeler VM, Kjorstad KE (1991) Endometrial adenocarcinoma in Norway. A study of a total population. Cancer 67 (12): 3093–3103.
Allory Y, Matsuoka Y, Bazille C, Christensen EI, Ronco P, Debiec H (2005) The L1 cell adhesion molecule is induced in renal cancer cells and correlates with metastasis in clear cell carcinomas. Clin Cancer Res 11 (3): 1190–1197.
Altevogt P, Doberstein K, Fogel M (2016) L1CAM in human cancer. Int J Cancer 138 (7): 1565–1576.
Beer S, Oleszewski M, Gutwein P, Geiger C, Altevogt P (1999) Metalloproteinase-mediated release of the ectodomain of L1 adhesion molecule. J Cell Sci 112 (Pt 16): 2667–2675.
Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, Angioli R, Tateo S, Mangili G, Katsaros D, Garozzo G, Campagnutta E, Donadello N, Greggi S, Melpignano M, Raspagliesi F, Ragni N, Cormio G, Grassi R, Franchi M, Giannarelli D, Fossati R, Torri V, Amoroso M, Croce C, Mangioni C (2008) Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 100 (23): 1707–1716.
Bondong S, Kiefel H, Hielscher T, Zeimet AG, Zeillinger R, Pils D, Schuster E, Castillo-Tong DC, Cadron I, Vergote I, Braicu I, Sehouli J, Mahner S, Fogel M, Altevogt P (2012) Prognostic significance of L1CAM in ovarian cancer and its role in constitutive NF-kappaB activation. Ann Oncol 23 (7): 1795–1802.
Boo YJ, Park JM, Kim J, Chae YS, Min BW, Um JW, Moon HY (2007) L1 expression as a marker for poor prognosis, tumor progression, and short survival in patients with colorectal cancer. Ann Surg Oncol 14 (5): 1703–1711.
Bosse T, Nout RA, Stelloo E, Dreef E, Nijman HW, Jurgenliemk-Schulz IM, Jobsen JJ, Creutzberg CL, Smit VT (2014) L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: pooled PORTEC trial results. Eur J Cancer 50 (15): 2602–2610.
Dellinger TH, Smith DD, Ouyang C, Warden CD, Williams JC, Han ES (2016) L1CAM is an independent predictor of poor survival in endometrial cancer - an analysis of The Cancer Genome Atlas (TCGA). Gynecol Oncol 141 (2): 336–340.
Eltabbakh GH, Shamonki J, Mount SL (2005) Surgical stage, final grade, and survival of women with endometrial carcinoma whose preoperative endometrial biopsy shows well-differentiated tumors. Gynecol Oncol 99 (2): 309–312.
Engelsen IB, Stefansson IM, Akslen LA, Salvesen HB (2008) GATA3 expression in estrogen receptor alpha-negative endometrial carcinomas identifies aggressive tumors with high proliferation and poor patient survival. Am J Obstet Gynecol 199 (5): 543 e1–543 e7.
Fogel M, Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Smirnov A, Edler L, Ben-Arie A, Huszar M, Altevogt P (2003) L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 362 (9387): 869–875.
Fons G, Hasibuan SM, van der Velden J, ten Kate FJ (2007) Validation of tissue microarray technology in endometrioid cancer of the endometrium. J Clin Pathol 60 (5): 500–503.
Frumovitz M, Singh DK, Meyer L, Smith DH, Wertheim I, Resnik E, Bodurka DC (2004) Predictors of final histology in patients with endometrial cancer. Gynecol Oncol 95 (3): 463–468.
Geels YP, Pijnenborg JM, Gordon BB, Fogel M, Altevogt P, Masadah R, Bulten J, van Kempen LC, Massuger LF (2016) L1CAM expression is related to non-endometrioid histology, and prognostic for poor outcome in endometrioid endometrial carcinoma. Pathol Oncol Res 22 (4): 863–868.
Gutwein P, Stoeck A, Riedle S, Gast D, Runz S, Condon TP, Marme A, Phong MC, Linderkamp O, Skorokhod A, Altevogt P (2005) Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clin Cancer Res 11 (7): 2492–2501.
Hua T, Liu S, Xin X, Jin Z, Liu Q, Chi S, Wang X, Wang H (2016) Prognostic significance of L1 cell adhesion molecule in cancer patients: a systematic review and meta-analysis. Oncotarget 7 (51): 85196–85207.
Huszar M, Pfeifer M, Schirmer U, Kiefel H, Konecny GE, Ben-Arie A, Edler L, Munch M, Muller-Holzner E, Jerabek-Klestil S, Abdel-Azim S, Marth C, Zeimet AG, Altevogt P, Fogel M (2010) Up-regulation of L1CAM is linked to loss of hormone receptors and E-cadherin in aggressive subtypes of endometrial carcinomas. J Pathol 220 (5): 551–561.
Kommoss F, Kommoss F, Grevenkamp F, Bunz AK, Taran FA, Fend F, Brucker SY, Wallwiener D, Schonfisch B, Greif K, Lax S, Staebler A, Kommoss S (2017) L1CAM: amending the ‘low-risk’ category in endometrial carcinoma. J Cancer Res Clin Oncol 143 (2): 255–262.
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4 (7): 844–847.
Krakstad C, Trovik J, Wik E, Engelsen IB, Werner HM, Birkeland E, Raeder MB, Oyan AM, Stefansson IM, Kalland KH, Akslen LA, Salvesen HB (2012) Loss of GPER identifies new targets for therapy among a subgroup of ERalpha-positive endometrial cancer patients with poor outcome. Br J Cancer 106 (10): 1682–1688.
Lampe B, Kurzl R, Hantschmann P (1995) Reliability of tumor typing of endometrial carcinoma in prehysterectomy curettage. Int J Gynecol Pathol 14 (1): 2–6.
Leitao MM Jr., Kehoe S, Barakat RR, Alektiar K, Gattoc LP, Rabbitt C, Chi DS, Soslow RA, Abu-Rustum NR (2008) Accuracy of preoperative endometrial sampling diagnosis of FIGO grade 1 endometrial adenocarcinoma. Gynecol Oncol 111 (2): 244–248.
Li Y, Galileo DS (2010) Soluble L1CAM promotes breast cancer cell adhesion and migration in vitro, but not invasion. Cancer Cell Int 10: 34.
Maggino T, Romagnolo C, Landoni F, Sartori E, Zola P, Gadducci A (1998) An analysis of approaches to the management of endometrial cancer in North America: a CTF study. Gynecol Oncol 68 (3): 274–279.
Maggino T, Romagnolo C, Zola P, Sartori E, Landoni F, Gadducci A (1995) An analysis of approaches to the treatment of endometrial cancer in western Europe: a CTF study. Eur J Cancer 31A (12): 1993–1997.
Maretzky T, Schulte M, Ludwig A, Rose-John S, Blobel C, Hartmann D, Altevogt P, Saftig P, Reiss K (2005) L1 is sequentially processed by two differently activated metalloproteases and presenilin/gamma-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol Cell Biol 25 (20): 9040–9053.
Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, Riedle S, Postina R, Fahrenholz F, Fogel M, Lemmon V, Altevogt P (2001) Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol 155 (4): 661–673.
Moos M, Tacke R, Scherer H, Teplow D, Fruh K, Schachner M (1988) Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature 334 (6184): 701–703.
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E (2016) Endometrial cancer. Lancet 387 (10023): 1094–1108.
Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, Graham JE (1991) Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 40 (1): 55–65.
Pecorelli S (2009) Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 105 (2): 103–104.
Rathjen FG, Schachner M (1984) Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J 3 (1): 1–10.
Raveh S, Gavert N, Ben-Ze’ev A (2009) L1 cell adhesion molecule (L1CAM) in invasive tumors. Cancer Lett 282 (2): 137–145.
Salvesen HB, Haldorsen IS, Trovik J (2012) Markers for individualised therapy in endometrial carcinoma. Lancet Oncol 13 (8): e353–e361.
Schafer MK, Altevogt P (2010) L1CAM malfunction in the nervous system and human carcinomas. Cell Mol Life Sci 67 (14): 2425–2437.
Schroder C, Schumacher U, Fogel M, Feuerhake F, Muller V, Wirtz RM, Altevogt P, Krenkel S, Janicke F, Milde-Langosch K (2009) Expression and prognostic value of L1-CAM in breast cancer. Oncol Rep 22 (5): 1109–1117.
Smogeli E, Davidson B, Cvancarova M, Holth A, Katz B, Risberg B, Kristensen G, Lindemann K (2016) L1CAM as a prognostic marker in stage I endometrial cancer: a validation study. BMC Cancer 16: 596.
Stefansson IM, Salvesen HB, Akslen LA (2004) Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol 22 (7): 1242–1252.
Tischler V, Pfeifer M, Hausladen S, Schirmer U, Bonde AK, Kristiansen G, Sos ML, Weder W, Moch H, Altevogt P, Soltermann A (2011) L1CAM protein expression is associated with poor prognosis in non-small cell lung cancer. Mol Cancer 10: 127.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65 (2): 87–108.
Trovik J, Wik E, Stefansson IM, Marcickiewicz J, Tingulstad S, Staff AC, Njolstad TS MoMaTec Study Group Vandenput I, Amant F, Akslen LA, Salvesen HB (2011) Stathmin overexpression identifies high-risk patients and lymph node metastasis in endometrial cancer. Clin Cancer Res 17 (10): 3368–3377.
Trovik J, Wik E, Werner HM, Krakstad C, Helland H, Vandenput I, Njolstad TS, Stefansson IM, Marcickiewicz J, Tingulstad S, Staff AC MoMaTEC Study Group Amant F, Akslen LA, Salvesen HB (2013) Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur J Cancer 49 (16): 3431–3441.
Tsutsumi S, Morohashi S, Kudo Y, Akasaka H, Ogasawara H, Ono M, Takasugi K, Ishido K, Hakamada K, Kijima H (2011) L1 Cell adhesion molecule (L1CAM) expression at the cancer invasive front is a novel prognostic marker of pancreatic ductal adenocarcinoma. J Surg Oncol 103 (7): 669–673.
van der Putten LJ, Visser NC, van de Vijver K, Santacana M, Bronsert P, Bulten J, Hirschfeld M, Colas E, Gil-Moreno A, Garcia A, Mancebo G, Alameda F, Trovik J, Kopperud RK, Huvila J, Schrauwen S, Koskas M, Walker F, Weinberger V, Minar L, Jandakova E, Snijders MP, van den Berg-van Erp S, Matias-Guiu X, Salvesen HB, Amant F, Massuger LF, Pijnenborg JM (2016) L1CAM expression in endometrial carcinomas: an ENITEC collaboration study. Br J Cancer 115 (6): 716–724.
Wang YY, Li L, Zhao ZS, Wang YX, Ye ZY, Tao HQ (2013) L1 and epithelial cell adhesion molecules associated with gastric cancer progression and prognosis in examination of specimens from 601 patients. J Exp Clin Cancer Res 32: 66.
Werner HM, Trovik J, Marcickiewicz J, Tingulstad S, Staff AC, Engh ME, Oddenes K, Rokne JA, Tjugum J, Lode MS, Amant F, Salvesen HB (2013) A discordant histological risk classification in preoperative and operative biopsy in endometrial cancer is reflected in metastatic risk and prognosis. Eur J Cancer 49 (3): 625–632.
Wojciechowski M, Glowacka E, Wilczynski M, Pekala-Wojciechowska A, Malinowski A (2017) The sL1CAM in sera of patients with endometrial and ovarian cancers. Arch Gynecol Obstet 295 (1): 225–232.
Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ (2012) Contemporary management of endometrial cancer. Lancet 379 (9823): 1352–1360.
Zander H, Rawnaq T, von Wedemeyer M, Tachezy M, Kunkel M, Wolters G, Bockhorn M, Schachner M, Izbicki JR, Kaifi J (2011) Circulating levels of cell adhesion molecule L1 as a prognostic marker in gastrointestinal stromal tumor patients. BMC Cancer 11 (189): 1–7.
Zeimet AG, Reimer D, Huszar M, Winterhoff B, Puistola U, Azim SA, Muller-Holzner E, Ben-Arie A, van Kempen LC, Petru E, Jahn S, Geels YP, Massuger LF, Amant F, Polterauer S, Lappi-Blanco E, Bulten J, Meuter A, Tanouye S, Oppelt P, Stroh-Weigert M, Reinthaller A, Mariani A, Hackl W, Netzer M, Schirmer U, Vergote I, Altevogt P, Marth C, Fogel M (2013) L1CAM in early-stage type I endometrial cancer: results of a large multicenter evaluation. J Natl Cancer Inst 105 (15): 1142–1150.
Acknowledgements
We thank the additional remaining MoMaTEC participants and their teams for patient inclusion and follow-up in their respective centres: Marie Ellstrøm Engh, Akershus University Hospital, Jostein Tjugum, Førde Hospital, Klaus Oddenes, Haugesund Hospital and Jan A Rokne, Tønsberg Hospital. This study was conducted within ENITEC (European Network for Individualized Treatment of Endometrial Cancer). We thank Ellen Valen, Britt Edvardsen and Kadri Madissoo for technical assistance. This study was supported by Helse Vest, the University of Bergen, the Norwegian Cancer Society, the Research Council of Norway and Bergen Research Foundation. Frédéric Amant is a senior researcher for the Research Fund-Flanders (FWO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Tangen, I., Kopperud, R., Visser, N. et al. Expression of L1CAM in curettage or high L1CAM level in preoperative blood samples predicts lymph node metastases and poor outcome in endometrial cancer patients. Br J Cancer 117, 840–847 (2017). https://doi.org/10.1038/bjc.2017.235
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.235
Keywords
This article is cited by
-
Preoperative pelvic MRI and 2-[18F]FDG PET/CT for lymph node staging and prognostication in endometrial cancer—time to revisit current imaging guidelines?
European Radiology (2022)
-
Patient-derived organoids reflect the genetic profile of endometrial tumors and predict patient prognosis
Communications Medicine (2021)
-
CD44, TGM2 and EpCAM as novel plasma markers in endometrial cancer diagnosis
BMC Cancer (2019)
-
Coffee and tea drinking in relation to the risk of differentiated thyroid carcinoma: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) study
European Journal of Nutrition (2019)