Abstract
Background:
Leiomyosarcoma is an aggressive soft tissue sarcoma with a 5-year survival rate of 15 to 60%. Treatment options for inoperable or metastatic patients are limited owing to frequent resistance of tumours to chemotherapy and radiation. In this study, we hypothesised that antiapoptotic Bcl-2 family proteins might contribute to leiomyosarcoma chemoresistance and therefore inhibition of Bcl-2 family proteins might sensitise leiomyosarcomas to conventional chemotherapy.
Methods:
Expression of the Bcl-2 family proteins Bcl-xL, Bcl-w and Bcl-2 was investigated using immunohistochemistry on a tissue microarray containing 43 leiomyosarcomas. Furthermore, we investigated whether ABT-737, a potent BH3 mimetic, sensitises leiomyosarcoma cells to doxorubicin treatment in vitro.
Results:
Seventy-seven per cent, 84% and 42% of leiomyosarcomas demonstrated high expression of Bcl-2, Bcl-xL and Bcl-w, respectively. Single-agent treatment with ABT-737 resulted in a minor reduction of cell viability. However, combination treatment of ABT-737 and doxorubicin revealed synergism in all four cell lines, by inducing apoptosis.
Conclusions:
In conclusion, Bcl-2 family proteins contribute to soft tissue leiomyosarcoma chemoresistance. Antiapoptotic proteins are highly expressed in leiomyosarcoma of soft tissue, and inhibition of these proteins using a BH3 mimetic increases leiomyosarcoma sensitivity to doxorubicin.
Similar content being viewed by others
Main
Leiomyosarcomas are malignant mesenchymal neoplasms displaying smooth muscle differentiation (Fletcher et al, 2013). They occur in patients over a wide age range with the highest incidence in the fifth and sixth decade of life. The 5-year survival rates range from 15 to 60% depending on the location, size and stage of disease at time of diagnosis (Fletcher et al, 2013). These low survival rates are in part attributable to low response rates to chemo- and radiotherapy (Villar et al, 2012).
Overexpression of multidrug resistance proteins, like P-glycoprotein, only partly explains leiomyosarcoma chemoresistance. In addition, overexpression of Bcl-2 family proteins could contribute to chemo- and radiotherapy resistance, since chemo- and radiotherapy are DNA-damaging therapies that provoke tumour regression by the activation of the intrinsic apoptotic pathway (Jiang and Wang, 2004; Abdullah and Chow, 2013; Tacar et al, 2013). Interestingly, immunohistochemistry shows high expression of the antiapoptotic protein Bcl-2 in soft tissue sarcomas, including leiomyosarcoma (Hong et al, 2001; Win et al, 2013). Bcl family members, like Bcl-2, Bcl-xL (BCL2L1) and Bcl-w (BCL2L2) inhibit activation of the intrinsic apoptotic pathway by regulating the mitochondrial outer membrane permeabilisation. Specifically, mitochondrial outer membrane pores are formed by dimerisation of Bax and Bak proteins. These pores release cytochrome c from mitochondria, which leads to activation of the intrinsic apoptotic cascade (Jiang and Wang, 2004). By binding of the antiapoptotic proteins Bcl-2/Bcl-xL to the proapoptotic family members Bak and Bax, the dimerisation of Bak and Bax is prevented and activation of the apoptotic pathway is inhibited.
In this study, we hypothesised that the Bcl-2 family members have an important role in leiomyosarcoma chemoresistance and their inhibition might therefore render leiomyosarcoma cells sensitive to treatment with conventional chemotherapeutic agents. To explore the role of Bcl-2 family members in leiomyosarcoma chemoresistance we first evaluated the protein expression of the antiapoptotic proteins Bcl-2, Bcl-xL and Bcl-w in a large series of soft tissue leiomyosarcomas by immunohistochemistry, using clinically annotated tissue microarrays. Subsequently, we determined whether the Bcl-2 pathway inhibitor ABT-737 sensitises leiomyosarcoma cells to chemotherapy. ABT-737 is a potent BH3 mimetic that inhibits Bcl-2, Bcl-xL and Bcl-w at the mitochondrial membrane by binding to their BH3 domains (Oltersdorf et al, 2005). As chemotherapeutic agent we used doxorubicin, which is widely used in the treatment of leiomyosarcoma (Grobmyer et al, 2004; Kraybill et al, 2006).
Materials and methods
Tissue samples
Tissue microarrays were previously constructed containing leiomyosarcomas (soft tissue n=40, uterine n=3), leiomyomas of the uterus (n=7), myxofibrosarcomas (n=16) and undifferentiated pleomorphic/spindle cell sarcomas (n=28). These sarcomas all belong to the genetic subcategory of soft tissue sarcomas with complex karyotypes. Tissue microarrays (TMAs) were constructed using a semi-automated TMA apparatus (TMA Master; 3D Histech, Budapest, Hungary) (de Graaff et al, 2013). For each case, 1.767 mm2 per core was present in triplicate. All samples were handled according to the Dutch code of proper secondary use of human material as accorded by the Dutch society of pathology (Federa). The samples were handled in a coded (pseudoanonymised) manner. All study methods were approved by the LUMC ethical board (B16.007). In total, 40 soft tissue leiomyosarcomas were included from 39 patients (23 females and 16 males); of one patient both the primary tumour and a metastatic lesion were analysed. In addition, three uterine leiomyosarcomas were included. The average age at diagnosis was 60 years. Clinicopathological information is shown in Supplementary Table 1. Patients were diagnosed between 1989 and 2011 in the LUMC (Leiden, The Netherlands). None of the patients received preoperative chemotherapy or radiation therapy.
Compounds
Doxorubicin was obtained from the in-house hospital pharmacy in a 0.9% NaCl solution. ABT-737 (Selleckchem, Houston, TX, USA) was dissolved in dimethyl sulfoxide.
Cell culture
In this study, we included four leiomyosarcoma cell lines (LMS04, LMS05, IB133 and IB140). These cell lines were established from primary tumours and for the experiments described herein were used between passages 20 and 50. LMS04 was derived from a high-grade retroperitoneal metastasis from the uterus and LMS05 was derived from a grade 2 soft tissue leiomyosarcoma (Supplementary Table 2). IB140 was derived from a FNCLCC grade 2 leiomyosarcoma from the upper leg and IB133 was derived from a FNCLCC grade 3 retroperitoneal leiomyosarcoma. TP53 mutation analysis was determined by Sanger sequencing. In addition, the cell lines were screened for hotspot mutations using the Ion AmpliSeq Cancer Hotspot panel v2 (Thermo Fisher Scientific, Bleiswijk, The Netherlands). Fifty reads were considered enough to draw reliable conclusions. The coverage of identified SNPs was ranging from 50 up to 1995 reads.
The leiomyosarcoma cell lines LMS04 and LMS05 were cultured in RPMI1640 medium with 15% FBS and 1% penicillin/streptomycin and the cell lines IB140 and IB133 were cultured in RPMI1640 with 10% FBS, 1% glutamax and 1% pen/strep. The HeLa cell line was included as a reference and cultured in RPMI1640 with 10% FBS and 1% pen/strep. All cell lines were repeatedly checked for mycoplasm and authentication of the cell lines was determined by short tandem repeat (STR) typing (GenePrint 10 system, Promega, Leiden, The Netherlands).
Immunohistochemistry
To detect the expression of antiapoptotic proteins, immunohistochemistry on TMA was performed according to standard laboratory procedures (de Graaff et al, 2013). Further details of the Bcl-2, Bcl-xL and Bcl-w antibodies are shown in Supplementary Table 3. Scoring was performed by two observers (MAdG and JVMGB) independently and discrepant cases were discussed to reach consensus. Both intensity (0=absent, 1=weak, 2=moderate and 3=strong expression) and percentage of positive tumour cells (0=0%, 1=1–24%, 2=25–49%, 3=50–74%, 4=75–100%) were assessed and scores were added up (Hoekstra et al, 2015).
Cell viability assays
To analyse the effect of treatment with doxorubicin and ABT-737, cell viability assays were performed with Alamar blue (Life Technologies, Bleiswijk, The Netherlands) according to the manufacturer’s instructions. Absorbance was measured with a light spectrometer (Victor V, 1420 Multilabel counter; Perkin Elmer, Groningen, The Netherlands).
The dose–response curves of single treatments with ABT-737 and doxorubicin were determined for each cell line in a range from 0.001 to 12.8 μ M. For doxorubicin, it is accepted that an in vivo range of 5 to 100 μ M corresponds to an in vitro range from 1 to 10 μ M (Shrivastav et al, 1980). All cell lines were treated with ABT-737 for 24 h and subsequently doxorubicin for 48 h. Because in only one of the four cell lines (LMS05) large effects could be observed, the other three cell lines were treated with doxorubicin and ABT-737 combined for 72 h. The concentrations of doxorubicin used for the combination treatments were selected per cell line, based on the individual dose–response curves after 48 h (not shown) and 72 h doxorubicin treatment. Cell viability was calculated relative to untreated cells, all experiments were repeated three times in triplicate.
To evaluate whether the combination treatments of doxorubicin and ABT-737 were synergistic drug interactions, a simplified version of the Bliss independence model was applied (Wong et al, 2012). When two drugs exert their effects independent to one another, the resulting relative viability after drug treatment is expected to equal the product of the relative viability after treatment with the individual drugs. When the observed viability after combination treatment is lower than the expected viability, this is an indication of a synergistic drug interaction. When the observed viability is higher than the expected based on the individual drug effects, the compounds inhibit each other’s effects (antagonism).
Western blot
To evaluate the expression of Bcl-2, Bcl-xL, Bcl-w, Mcl-1, PARP and α-tubulin before and after treatment we performed western blots according to the Cell Signaling Technology (Leiden, The Netherlands) protocol enhanced chemiluminescence. Cell lysates were made from cultured cell lines LMS04, LMS05, IB133 and IB140 treated with doxorubicin (respectively, 0.2, 0.6, 0.7 and 0.5 μ M) and with ABT-737 (5 μ M) for 24 h. In order to obtain cell lysates, hot SDS was added to the cells and the solution was heated up to 100 °C. Gels were loaded with 20 μg lysate. Antibodies, described in detail in Supplementary Table 4, were dissolved in TPS Tween 0.05% in which the blots were also washed. As a positive control for all antibodies except for PARP, the HeLa cell line was included. For PARP, Jurkat apoptosis cell lysate (Cell Signaling Technology) was included as a positive control.
Caspase assay
To confirm that the decrease in cell viability was caused by apoptosis, caspase assays were performed according to the caspase-Glo 3/7 assay protocol of Promega. LMS04, LMS05, IB133 and IB140 cell lines were treated for 24 h with ABT-737 (5 μ M), doxorubicin (0.2, 0.6, 0.7 and 0.5 μ M, respectively) or a combination. A 1 : 1 ratio of Caspase-Glo 3/7 (Promega) reagent volume per sample was used. As negative controls, untreated cells were included. For each condition, Z-VAD-FMK, an irreversible pan-caspase inhibitor (25 μ M, Promega) was used. The luciferase units of each sample were measured by the VICTOR3 Label counter with emission filter D572 after 1 h.
Statistics
GraphPad Prism for Windows version 6.02 (GraphPad Software, La Jolla, CA, USA) was used to analyse the results. The statistical difference between 2 groups was assessed by an unpaired t-test, P-values were considered statistically different if P<0.05. Statistical differences in overall survival were calculated with the log-rank (Mantel–Cox) test. Also GraphPad was used for dose–response curves and calculation of IC50 values.
Results
High expression of Bcl family proteins in leiomyosarcomas
Immunohistochemistry for antiapoptotic proteins using tissue microarrays revealed that Bcl-xL and Bcl-w were higher expressed in leiomyosarcomas compared with uterine leiomyomas (Figure 1). Thirty three out of 43 (77%) leiomyosarcomas showed high Bcl-2 expression (IHC score>3). For Bcl-xL and Bcl-w this was 36 out of 43 (84%) and 18 out of 43 (42%), respectively (Table 1). Interestingly, Bcl-xL and Bcl-w were expressed at significantly lower levels in uterine leiomyomas (P⩽0.001), while Bcl-2 expression was significantly higher in leiomyomas, compared with leiomyosarcomas (P=0.042). The three uterine leiomyosarcomas showed a slightly higher Bcl-2 expression compared with the leiomyomas, although this is not statistically significant (P=0.224) (Supplementary Figure 1A). The antiapoptotic proteins were also expressed in other high-grade sarcomas (myxofibrosarcoma and undifferentiated pleomorphic sarcoma) (Table 1).
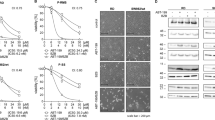
Protein expression of Bcl-2, Bcl-xL and Bcl-w determined by immunohistochemistry.(A) In the top panel, Bcl-2, Bcl-xL and Bcl-w expression of a representative leiomyosarcoma is displayed were the bottom panel shows expression of a representative uterine leiomyoma (× 20 magnification). Leiomyosarcomas showed overall higher expression of Bcl-xL and Bcl-w, whereas both leiomyosarcomas and uterine leiomyomas revealed a high expression of Bcl-2. (B) The difference in Bcl-2, Bcl-xL and Bcl-w protein expression of leiomyosarcoma (LMS), uterine leiomyoma (LM uterus), myxofibrosarcoma (MFS) and undifferentiated pleomorphic sarcoma (UPS) illustrated by dot plots confirmed an overall higher expression of Bcl-xL and Bcl-w in leiomyosarcomas as compared with uterine leiomyomas.
There was no association between expression and histological grade (Supplementary Figure 1A). The Kaplan–Meier survival curve showed a trend for high expression of Bcl-2, Bcl-xL and Bcl-w to be associated with shorter overall survival time (Supplementary Figure 1B). However, the differences in overall survival between the groups of patients with an immunohistochemical score ⩽3 or >3 were not significant (P=0.5347 (Bcl-2), P=0.5490 (Bcl-xL) and P=0.2495 (Bcl-w)). Sixteen patients were diagnosed with a metastasis after a period of treatment. In the Kaplan–Meier survival curves no correction was added for metastasis or treatment.
Doxorubicin and ABT-737 act synergistically in leiomyosarcoma cell lines
Since Bcl-2, Bcl-xL and Bcl-w were highly expressed in the majority of leiomyosarcomas, four leiomyosarcomas cell lines (LMS04, LMS05, IB133 and IB140) were selected for further functional evaluation. Clinicopathological as well as genetic characteristics of these cell lines are shown in Supplementary Table 2. TP53 mutations were found in LMS04 and IB133, while IB140 demonstrated a CTNNB1 variation (Supplementary Table 2 and Supplementary Figure 2).
The response to ABT-737 and doxorubicin were determined separately after 24, 48 and 72 h of treatment. All cell lines showed maximal response at 72 h of ABT-737 treatment. IB140 and IB133 demonstrated a slight decrease in cell viability compared with LMS04 and LMS05, however due to the limited sensitivity of single treatment with this drug, no reliable absolute IC50 could be calculated (Figure 2A). The cell lines showed a maximal reduction in cell viability of 60–95% after single doxorubicin treatment (Figure 2B). The calculated absolute IC50 values of doxorubicin treatment for the four cell lines LMS04, LMS05, IB140 and IB133 were respectively: 0.198; 0.265; 1.314 and 0.580 μ M.
Dose–response curves of the four cell lines treated with ABT-737 and doxorubicin.(A) Dose–response curves of ABT-737 ranging from 0.001–12.8 μ M after 72 h of treatment showed limited effect of this single drug treatment. (B) Dose–response curves of doxorubicin ranging from 0.001–12.8 μ M after 72 h of treatment showed reductions till 5% cell viability. (C) Comparison of observed and expected relative cell viability of LMS05 after sequential treatment with ABT-737 for 24 h followed by 48 h treatment with doxorubicin. Strong synergism was seen in LMS05. (D–F) Comparison of observed and expected relative cell viability of the cell lines LMS04, IB140 and IB133 after synchronous combination treatment with ABT-737 and doxorubicin for 72 h. A moderate synergistic effect was observed in IB140 and IB133, and LMS04 revealed a mild synergistic effect at ABT-737 10 μ M.
To determine possible synergy of combination treatment, ABT-737 (1, 5 and 10 μ M) was administered for the first 24 h, followed by the addition of doxorubicin for 48 h. A synergistic effect of ABT-737 was found, most pronounced in LMS05 (Figure 2C) and only a moderate effect was observed in IB140, IB133 and LMS04 (not shown). Therefore, these last three cell lines were treated simultaneously with ABT-737 and doxorubicin for 72 h. The expected cell viability of combination treatment was calculated and compared with the observed viability. For five out of six tested sequential drug combinations in LMS05 a statistical significant difference (P<0.005) was found between the expected and observed cell viability, indicating synergism (Figure 2C). IB133 and IB140 both showed synergistic effects with pronounced reduction of cell viability in the three drug combinations with the higher doxorubicin concentration (P<0.005) after synchronous treatment for 72 h (Figure 2E and F). In LMS04, on the other hand, the difference between the observed and expected effect of the synchronous combination treatment was most clear in the two combinations with the highest (10 μ M) ABT-737 concentration (P=0.07 and P<0.005; Figure 2D).
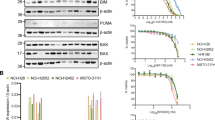
Western blot performed on untreated cells confirmed expression of all three antiapoptotic proteins in the four cell lines. Among these, Bcl-xL was most abundantly expressed. Expression of Bcl-2 was highest in LMS05, followed by LMS04, while expression was almost absent in IB133 and IB140 (Figure 3). Mcl-1 was expressed in all cell lines, with a comparable intensity as the HeLa control. LMS04 even showed a stronger Mcl-1 expression. Treatment with ABT-737 and/or doxorubicin did not significantly alter the expression levels of Mcl-1, Bcl-w, Bcl-xL or Bcl-2.
Western blots performed on untreated, single-agent-treated or combination-agent-treated cells.The expression of Mcl-1, Bcl-w, Bcl-xL, α-tubulin and Bcl-2 in four leiomyosarcoma cell lines showed that Bcl-2 was expressed in LMS05 and LMS04, whereas it was almost absent in the IB133 and IB140 cell lines. Bcl-xL was most abundantly expressed in all four cell lines. Mcl-1 was expressed in all cell lines.
Combination treatment induces apoptosis
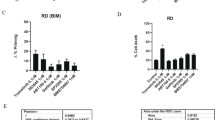
Using caspase Glo3/7 assays we demonstrated that reductions in cell viability were caused by apoptosis. All four cell lines showed a marked increase in caspase activity after combination treatment compared with single treatment (Figure 4A). Addition of the pan-caspase inhibitor Z-VAD-FMK blocked the increase in luciferase units, which indicates that the luciferase units correspond to apoptosis. Furthermore, induction of cleaved PARP, an indicator for apoptosis was greater in all 24 h combination treated lysates, compared with the single-agent-treated or untreated samples (Figure 4B; Shrivastav et al, 1980).
Caspase Glo3/7 assay and PARP western blots to evaluate apoptosis after single-agent and combination treatments.(A) Amount of luciferase units measured in combined treated cells was significantly higher compared with single and untreated cells. For each condition also the pan-caspase inhibitor (Z-VAD-FMK) was included, which show low or no expression of luciferase units. (B) Cleaved PARP expression was confirmed in all combined treated cells by western blot.
Discussion
In this study, we found high expression of Bcl-2 family members in leiomyosarcomas and demonstrate a role for these proteins in the response of leiomyosarcomas to conventional chemotherapy. More specifically, inhibition of Bcl-2 family members using the ABT-737 BH3 mimetic rendered leiomyosarcoma cell lines more sensitive to doxorubicin, suggesting new therapeutic options for patients with metastatic or inoperable disease.
We demonstrated a high protein expression of Bcl-xL and Bcl-w in leiomyosarcomas compared to benign uterine smooth muscle tumours. Bcl-2 expression was higher in leiomyomas compared to leiomyosarcomas, which was also reported by others (Zhai et al, 1999; Bodner et al, 2004). Some studies showed a correlation between high Bcl-2 expression and longer disease-specific survival in uterine leiomyosarcomas (Zhai et al, 1999; Bodner et al, 2004; Leiser et al, 2006; Lusby et al, 2013). In contrast, we observed a trend for high Bcl-2 expression with shorter survival. The difference might be due to differences in biological behaviour between uterine and soft tissue leiomyosarcomas as our series contains only three uterine leiomyosarcomas.
These results are consistent with observations in other tumour types, including sarcomas, in which in vitro studies have also demonstrated an important role for the Bcl-2 family members in chemoresistance, and that tumour cells could be sensitised to doxorubicin by inhibition of these proteins, using ABT-737 (van Oosterwijk et al, 2012). The addition of doxorubicin after ABT-737 treatment resulted in a complete loss of cell viability in chondrosarcoma. In other tumour cell lines, including castration-resistant prostate cancer, malignant peripheral nerve sheath tumour, acute promyelocytic leukaemia and acute myeloid leukaemia, ABT-737 also showed synergy in combination with chemotherapeutic agents (Ugarenko et al, 2010; Lee et al, 2012; Parrondo et al, 2013; Baev et al, 2014).
Treatment with ABT-737 alone resulted only at a high concentration in a small reduction of the cell viability in the IB133 and IB140 cell lines. Therefore, we concluded that in vitro treatment with ABT-737 as single agent has almost no influence on the cell viability. Other studies in chondrosarcomas and small cell lung carcinomas also revealed limited activity of ABT-737 monotherapy (Rudin et al, 2012). To explore a possible role of the Bcl-2 family members in response to conventional chemotherapy, we combined ABT-737 with doxorubicin, which is commonly used as single agent in the treatment of high-grade soft tissue sarcomas (Grobmyer et al, 2004; Kraybill et al, 2006). Indeed, a synergistic effect was seen as cell viability decreased and apoptosis could be shown. Interestingly, single treatment with doxorubicin decreased cell viability, but had no impact on apoptosis. This suggests that even though cells stopped proliferating and lost their viability, they failed to activate their apoptotic machinery at the doses tested, most probably due to expression of Bcl-2 family members. This is in accordance with the low response rates and the poor impact of chemotherapeutic agents on overall survival of patients.
The four leiomyosarcoma cell lines were analysed using the AmpliSeq Cancer Hotspot Panel v2. We detected a CTNNB1 S45C variation in IB140, with a coverage of 61 reads. Revision of the corresponding primary tumour confirmed the diagnosis of leiomyosarcoma as well as the presence of the same mutation (Supplementary Figure 2). Nuclear staining for β-catenin was absent, so the significance of the S45C CTNNB1 alteration in this tumour remains unclear. Two cell lines showed alterations in TP53. The leiomyosarcoma cell lines that showed the highest synergy (LMS05 and IB140) have a wild-type TP53 gene, whereas the cell line with the lowest synergy (LMS04) did not. TP53 is a major protein in the apoptotic signalling pathway and when TP53 is mutated the apoptotic response is impaired (Levine, 1997). Furthermore, wild-type TP53 increases the responsiveness to chemotherapy by downregulating multidrug resistance-1 expression (Zhan et al, 2001). Even though the numbers are small, a mutation in this key protein might explain the difference in treatment response for LMS04 and IB133.
Furthermore, the cell lines showed Mcl-1 expression at the same level or stronger as compared with the HeLa control, and it has been reported that Mcl-1 in HeLa cells has an important role in the sensitisation to ABT-737 (van Delft et al, 2006). High Mcl-1 levels are associated with resistance of cell lines to ABT-737 and downregulation of Mcl-1 sensitises carcinoma cells to doxorubicin (Konopleva et al, 2006; van Delft et al, 2006; Long et al, 2015). This suggests that Mcl-1 expression might be important in the antiapoptotic mechanisms in the LMS cell lines, and modulation of this protein level might be a target to increase the effect of ABT-737 and doxorubicin treatment. Treatment with ABT-737, doxorubicin or a combination did not change the expression levels of Bcl-xL, Bcl-w or Bcl-2. This is comparable to other studies in which there were only slight differences in the expression of Bcl-2 and Bcl-xL after treatment (Rooswinkel et al, 2012; Parrondo et al, 2013). Thus, ABT-737 and doxorubicin do not downregulate the expression of the Bcl-2 family members at the protein level, and may instead block their inhibitory effect on Bak/Bax proteins in the mitochondrial membrane (DeMatteo et al, 2000).
The effect of ABT-263 (navitoclax, Abbott, Lake Forest, IL, USA), a structural analogue of ABT-737, has been analysed in toxicity phase I and II studies in small cell lung carcinomas. Thrombocytopenia was the most frequently observed adverse effect. However, marrow compensation platelet counts showed partial recovery when dosing was sustained, and in the week without dosing, recovery to baseline was seen. Diarrhoea, vomiting, nausea and fatigue were other common adverse effects (Gandhi et al, 2011; Rudin et al, 2012). These adverse events are also frequently seen after chemotherapeutic treatment but are most of the time not life threatening, suggesting that the preclinical evidence generated in this report may be realistically translated to clinical application.
In conclusion, our results show that inhibition of Bcl-2 family members using ABT-737 renders leiomyosarcoma cell lines more sensitive to doxorubicin, especially in the presence of wild-type TP53, suggesting new potential therapeutic options for leiomyosarcoma patients with metastatic or inoperable disease.
Change history
24 May 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abdullah LN, Chow EK (2013) Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med 2 (1): 3.
Baev DV, Krawczyk J, ODwyer M, Szegezdi E (2014) The BH3-mimetic ABT-737 effectively kills acute myeloid leukemia initiating cells. Leuk Res Rep 3 (2): 79–82.
Bodner K, Bodner-Adler B, Kimberger O, Czerwenka K, Mayerhofer K (2004) Bcl-2 receptor expression in patients with uterine smooth muscle tumors: an immunohistochemical analysis comparing leiomyoma, uterine smooth muscle tumor of uncertain malignant potential, and leiomyosarcoma. J Soc Gynecol Investig 11 (3): 187–191.
de Graaff MA, Cleton-Jansen AM, Szuhai K, Bovee JVMG (2013) Mediator complex subunit 12 exon 2 mutation analysis in different subtypes of smooth muscle tumors confirms genetic heterogeneity. Hum Pathol 44 (8): 1597–1604.
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231 (1): 51–58.
Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (2013) World Health Organisation Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, France.
Gandhi L, Camidge DR, Ribeiro de OM, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM, Dive C, Enschede SH, Nolan C, Chiu YL, Busman T, Xiong H, Krivoshik AP, Humerickhouse R, Shapiro GI, Rudin CM (2011) Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 29 (7): 909–916.
Grobmyer SR, Maki RG, Demetri GD, Mazumdar M, Riedel E, Brennan MF, Singer S (2004) Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol 15 (11): 1667–1672.
Hoekstra AS, de Graaff MA, Briaire-de Bruijn IH, Ras C, Seifar RM, van MI, Cornelisse CJ, Hogendoorn PC, Breuning MH, Suijker J, Korpershoek E, Kunst HP, Frizzell N, Devilee P, Bayley JP, Bovee JVMG (2015) Inactivation of SDH and FH cause loss of 5hmC and increased H3K9me3 in paraganglioma/pheochromocytoma and smooth muscle tumors. Oncotarget 6 (36): 38777–38788.
Hong T, Shimada Y, Uchida S, Itami A, Li Z, Ding Y, Kaganoi J, Komoto I, Sakurai T, Imamura M (2001) Expression of angiogenic factors and apoptotic factors in leiomyosarcoma and leiomyoma. Int J Mol Med 8 (2): 141–148.
Jiang X, Wang X (2004) Cytochrome C-mediated apoptosis. Annu Rev Biochem 73: 87–106.
Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M (2006) Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10 (5): 375–388.
Kraybill WG, Harris J, Spiro IJ, Ettinger DS, DeLaney TF, Blum RH, Lucas DR, Harmon DC, Letson GD, Eisenberg B (2006) Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol 24 (4): 619–625.
Lee SJ, Park HJ, Kim YH, Kim BY, Jin HS, Kim HJ, Han JH, Yim H, Jeong SY (2012) Inhibition of Bcl-xL by ABT-737 enhances chemotherapy sensitivity in neurofibromatosis type 1-associated malignant peripheral nerve sheath tumor cells. Int J Mol Med 30 (2): 443–450.
Leiser AL, Anderson SE, Nonaka D, Chuai S, Olshen AB, Chi DS, Soslow RA (2006) Apoptotic and cell cycle regulatory markers in uterine leiomyosarcoma. Gynecol Oncol 101 (1): 86–91.
Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88 (3): 323–331.
Long J, Ji Z, Jiang K, Wang Z, Meng G (2015) miR-193b modulatess resistance to doxorubicin in human breast cancer cells by downregulating MCL-1. Biomed Res Int 2015: 373574.
Lusby K, Savannah KB, Demicco EG, Zhang Y, Ghadimi MP, Young ED, Colombo C, Lam R, Dogan TE, Hornick JL, Lazar AJ, Hunt KK, Anderson ML, Creighton CJ, Lev D, Pollock RE (2013) Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution's experience. Ann Surg Oncol 20 (7): 2364–2372.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435 (7042): 677–681.
Parrondo R, de Las PA, Reiner T, Perez-Stable C (2013) ABT-737, a small molecule Bcl-2/Bcl-xL antagonist, increases antimitotic-mediated apoptosis in human prostate cancer cells. PeerJ 1: e144.
Rooswinkel RW, van de Kooij B, Verheij M, Borst J (2012) Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1, Bfl-1, or Bcl-B. Cell Death Dis 3: e366.
Rudin CM, Hann CL, Garon EB, Ribeiro de OM, Bonomi PD, Camidge DR, Chu Q, Giaccone G, Khaira D, Ramalingam SS, Ranson MR, Dive C, McKeegan EM, Chyla BJ, Dowell BL, Chakravartty A, Nolan CE, Rudersdorf N, Busman TA, Mabry MH, Krivoshik AP, Humerickhouse RA, Shapiro GI, Gandhi L (2012) Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 18 (11): 3163–3169.
Shrivastav S, Bonar RA, Stone KR, Paulson DF (1980) An in vitro assay procedure to test chemotherapeutic drugs on cells from human solid tumors. Cancer Res 40 (12): 4438–4442.
Tacar O, Sriamornsak P, Dass CR (2013) Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 65 (2): 157–170.
Ugarenko M, Nudelman A, Rephaeli A, Kimura K, Phillips DR, Cutts SM (2010) ABT-737 overcomes Bcl-2 mediated resistance to doxorubicin-DNA adducts. Biochem Pharmacol 79 (3): 339–349.
van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DC (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10 (5): 389–399.
van Oosterwijk JG, Herpers B, Meijer D, Briaire-de Bruijn IH, Cleton-Jansen AM, Gelderblom H, van de Water B, Bovee JVMG (2012) Restoration of chemosensitivity for doxorubicin and cisplatin in chondrosarcoma in vitro: BCL-2 family members cause chemoresistance. Ann Oncol 23 (6): 1617–1626.
Villar VH, Vogler O, Martinez-Serra J, Ramos R, Calabuig-Farinas S, Gutierrez A, Barcelo F, Martin-Broto J, Alemany R (2012) Nilotinib counteracts P-glycoprotein-mediated multidrug resistance and synergizes the antitumoral effect of doxorubicin in soft tissue sarcomas. PLoS One 7 (5): e37735.
Win TT, Yusuf Y, Jaafar H (2013) Apoptotic activities in soft tissue sarcoma: immunohistochemical study and their association with tumour characteristics. Malays J Med Sci 20 (2): 10–16.
Wong M, Tan N, Zha J, Peale FV, Yue P, Fairbrother WJ, Belmont LD (2012) Navitoclax (ABT-263) reduces Bcl-x(L)-mediated chemoresistance in ovarian cancer models. Mol Cancer Ther 11 (4): 1026–1035.
Zhai YL, Nikaido T, Toki T, Shiozawa A, Orii A, Fujii S (1999) Prognostic significance of bcl-2 expression in leiomyosarcoma of the uterus. Br J Cancer 80 (10): 1658–1664.
Zhan M, Yu D, Lang A, Li L, Pollock RE (2001) Wild type p53 sensitizes soft tissue sarcoma cells to doxorubicin by down-regulating multidrug resistance-1 expression. Cancer 92 (6): 1556–1566.
Acknowledgements
The authors thank Inge Briaire-de Bruijn and Yvonne de Jong for expert technical assistance. Financial support: Institut Bergonie and the Leiden University Medical Center are partners in Eurosarc and the research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no 278742 (Eurosarc).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
de Graaff, M., de Rooij, M., van den Akker, B. et al. Inhibition of Bcl-2 family members sensitises soft tissue leiomyosarcomas to chemotherapy. Br J Cancer 114, 1219–1226 (2016). https://doi.org/10.1038/bjc.2016.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.117
Keywords
This article is cited by
-
BCL-w: apoptotic and non-apoptotic role in health and disease
Cell Death & Disease (2020)
-
RAB25 confers resistance to chemotherapy by altering mitochondrial apoptosis signaling in ovarian cancer cells
Apoptosis (2020)
-
Bcl-xL inhibition enhances Dinaciclib-induced cell death in soft-tissue sarcomas
Scientific Reports (2019)
-
Maackia amurensis agglutinin induces apoptosis in cultured drug resistant human non-small cell lung cancer cells
Glycoconjugate Journal (2019)
-
High nuclear expression of proteasome activator complex subunit 1 predicts poor survival in soft tissue leiomyosarcomas
Clinical Sarcoma Research (2016)