Abstract
Cellular senescence is an established tumour-suppressive mechanism that prevents the proliferation of premalignant cells. However, several lines of evidence show that senescent cells, which often persist in vivo, can also promote tumour progression in addition to other age-related pathologies via the senescence-associated secretory phenotype (SASP). Moreover, new insights suggest the SASP can facilitate tissue repair. Here, we review the beneficial and detrimental roles of senescent cells, highlighting conditions under which the senescence response does and does not promote pathology, particularly cancer. By better understanding the context-dependent effects of cellular senescence, it may be feasible to limit its detrimental properties while preserving its beneficial effects, and develop novel therapeutic strategies to prevent or treat cancer and possibly other age-associated diseases.
Similar content being viewed by others
Main
Cellular senescence was first identified as an intrinsic process that halts the proliferation of normal cells (Hayflick, 1965; Campisi, 2005). Decades later, it became apparent that the senescence response is a potent tumour-suppressive mechanism that halts the proliferation of premalignant cells (Hayflick, 1965; Campisi, 2005). Mice (and humans) that are deficient in mounting a senescence response are inevitably cancer prone.
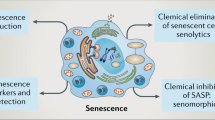
In response to the potentially oncogenic signals, cells enter an essentially irreversible senescent state, which is established and maintained by the p53/p21 and p16INK4a/pRB tumour-suppressor pathways (Serrano et al, 1997). Ironically, the accumulation of senescent cells over time can also promote tumorigenesis through the secretion of numerous bioactive molecules termed as the senescence-associated secretory phenotype (SASP; Coppe et al, 2008) or senescence-messaging secretome (Kuilman and Peeper, 2009) (Figure 1). SASP factors include pro-inflammatory cytokines, chemokines, growth factors and proteases (Kortlever et al, 2006; Acosta et al, 2008; Wajapeyee et al, 2008). Although the SASP can contribute to a pro-carcinogenic microenvironment, recent findings show it can also promote tissue remodelling and wound healing (Demaria et al, 2014). Therefore, depending upon the context, it is now clear that cellular senescence and the SASP contribute to myriad physiological functions, both beneficial and deleterious. The goal of this review is to present and discuss these different aspects.
Bright and dark sides of cellular senescence.The bright side (left). Senescence growth arrest prevents tumorigenesis (senescence growth arrest effects=blue arrows). Senescence also limits fibrosis by preventing proliferation of cells that secrete ECM, whereas the SASP includes matrix metalloproteinases that digest fibrotic lesions (SASP effects=red arrows). Certain features of normal embryonic development are promoted by senescent cells, though it is unclear if this is dependent on the SASP. Components of the SASP accelerate wound closure and attract immune cells. The dark side (right). The loss of proliferative potential that accompanies senescence impairs tissue regeneration and promotes aging, whereas the SASP also promotes aging at least in part by inducing a chronic inflammatory state in the tissue microenvironment. The SASP also contains factors that promote angiogenesis, cell proliferation, and cancer cell invasiveness. Furthermore, immune cells attracted by the SASP can disrupt the local microenvironment and promote tumour cell invasion. These final activities result in cancer progression.
The ‘bright side’ of senescence and the SASP
As mentioned in the Introduction, senescence was initially considered as a tumour-suppressive mechanism (Hayflick, 1965), and this aspect has been the subject of a substantial amount of literature during the past several decades. However, it is only recently that other beneficial effects of senescent cells have been described, and it is on these novel aspects that we are focusing the first part of our review.
Senescent cells facilitate tissue repair
In addition to stopping proliferation of premalignant cells, some data suggest that senescence has indeed other beneficial effects. In response to acute liver injury, hepatic stellate cells are induced to proliferate and produce extracellular matrices to repair the damage, but then undergo senescence. In mice lacking senescence effectors, hepatic stellate cells continue to proliferate and produce excessive extracellular matrix (ECM) leading to tissue fibrosis. Thus, liver injury induces a senescence response, which limited liver fibrosis; this effect is critically dependent on the matrix metalloproteinases that comprise part of the SASP (Krizhanovsky et al, 2008b). In the presence of chronic liver damage, ablation of a p53-dependent senescence programme in hepatic stellate cells increases liver fibrosis and cirrhosis, and this ablation is associated with reduced survival and enhanced transformation of adjacent epithelial cells into hepatocellular carcinoma (Lujambio et al, 2013).
Although the limitation of hepatic fibrosis is an important consequence of senescence, other models of tissue injury reveal additional roles for senescent cells in response to injury. Indeed, senescent cells and the SASP are also important for optimal wound healing (Jun and Lau, 2010; Demaria et al, 2014). In response to cutaneous wounds, fibroblasts and endothelial cells undergo senescence in a matricellular CCN1-dependent manner (Jun and Lau, 2010). This senescence accelerates wound closure by inducing myofibroblast differentiation through the secretion of platelet-derived growth factor AA (PDGF-AA; Demaria et al, 2014). Selective elimination of p16INK4a-positive senescent cells using transgenic mice delays wound closure, and topical treatment of senescence-free wounds with recombinant PDGF-AA rescues the delayed wound closure and induces myofibroblast differentiation in the absence of senescence.
Senescent cells recruit immune cells
In addition to its beneficial role in wound healing, senescent cells recruit innate immune cells to kill tumour cells (Ventura et al, 2007; Xue et al, 2007). In particular, SASP factors recruit natural killer cells to eliminate malignant cells (Iannello et al, 2013). In addition, SASP components attract immune cells, which can remove nearby damaged cells (Krizhanovsky et al, 2008a). Premalignant but senescent murine hepatocytes secrete chemokines and cytokines that promote immune-mediated clearance (senescence surveillance), which requires an intact CD4+ T-cell-mediated adaptive immune response. Impaired immune surveillance of these premalignant senescent hepatocytes fosters the development of hepatocellular carcinomas (Kang et al, 2011). Thus, senescence can also suppress tumorigenesis in vivo through immune surveillance (Figure 1).
Senescence in development
Finally, senescent cells are also important during embryonic development for fine-tuning the morphogenesis of certain structures (Munoz-Espin et al, 2013; Storer et al, 2013). Senescence was only partially compensated for by apoptosis, thus attributing a role for senescence during embryonic tissue development. In contrast to damage and injury-induced senescence, embryonic senescence was found to be p21-dependent, but p53- and p16INK4a-independent.
Thus, there is a beneficial role for senescence and the SASP in development and tissue repair—an important ‘bright side’ of senescence that extends beyond tumour suppression (Figure 1). However, evidence continues to mount that the SASP can also be detrimental.
The ‘dark side’ of senescence and the SASP
The SASP is pro-tumorigenic
The SASP can create a pro-tumorigenic microenvironment in several ways. First, SASP factors support tumour cell invasion and metastasis by disrupting and remodelling tissue structure (Coppe et al, 2008; Rodier and Campisi, 2011). Senescent cells secrete large amounts of proteases that degrade the ECM, rendering the tissue structure more relaxed and thus facilitating the invasion of cancer cells (Coppe et al, 2008). SASP factors also facilitate tumour cell invasion by inducing an epithelial-to-mesenchymal transition in neighbouring cells (Laberge et al, 2012a). Specific interleukins that are part of the SASP indeed contribute to connect senescence with an inflammatory phenotype and cancer (Kuilman et al, 2008). Very recently, Baker et al (2016) demonstrated that the clearance of p16INK4a-positive cells delays tumorigenesis and attenuates age-related deterioration of several organs.
Second, senescent cells can directly or indirectly promote tumour vascularisation. Senescent cells secrete a variety of angiogenic factors that promote the proliferation and assembly of endothelial cells for neo-angiogenesis (Davalos et al, 2010). Senescent cells can also indirectly promote angiogenesis by recruiting macrophages and stimulating them to adopt a pro-angiogenic M2 phenotype (Kelly et al, 2007).
Third, the SASP promotes tumour growth by establishing a microenvironment that is immunosuppressive. Senescent cells secrete cytokines that recruit myeloid-derived suppressor cells that inhibit CD8+ T-lymphocyte-mediated killing of tumour cells (Toso et al, 2014). By modifying the SASP through STAT3 inhibition, the secretion of several immunosuppressive cytokines is diminished leading to a strong anti-tumour response triggered by CD8+ T lymphocytes and tumour regression (Toso et al, 2014). These various activities of the SASP (Figure 1) make it an appealing target for novel adjuvant anti-cancer therapies.
Methods to target the detrimental SASP
The SASP can be targeted using several approaches. First, the SASP can be eliminated by directly killing the senescent cells, particularly using genetically modified mice. The selective elimination of p16INK4a-expressing senescent cells by apoptosis delays several age-related pathologies, in a progeroid mouse model (Baker et al, 2011; Childs et al, 2014), and tumorigenesis (Baker et al, 2016). Another recent study showed that hematopoetic cells were rejuvenated following elimination of senescent cells in mice prematurely aged due to total body irradiation (Chang et al, 2015). Another way to target the SASP is by decreasing the secretion of SASP factors. One approach is an inhibition of p38MAPK (Freund et al, 2011). Diverse stimuli activate this stress-inducible kinase when normal cells undergo senescence, and p38MAPK inhibition by genetic or pharmacological means markedly reduces the secretion of many SASP factors.
From a translation perspective, the SASP can also be targeted in a purely pharmacological approach using specific compounds. For example, treatment of human fibroblasts with glucocorticoids—corticosterone or cortisol—suppresses senescence-associated inflammation without reversing the tumour-suppressive growth arrest, in both oncogene- and radiation-induced senescent cells (Laberge et al, 2012b). Similarly, the mTOR inhibitor, rapamycin, blunts the pro-inflammatory phenotype of senescent human fibroblasts by preventing the translation of IL-1α, which controls the expression of other pro-inflammatory cytokines such as IL-6 (Orjalo et al, 2009; Laberge et al, 2015). Importantly, rapamycin suppresses the ability of senescent fibroblasts to stimulate prostate tumour growth in mice (Laberge et al, 2015). Furthermore, simvastatin, an approved lipid-regulating drug, was recently shown to decrease the SASP of senescent human fibroblasts by inhibiting protein prenylation without affecting the growth arrest (Liu et al, 2015). The Rho GTPases Rac1 and Cdc42 are activated in senescent cells, and simvastatin reduces both activities leading to reduced secretion of the major SASP factor IL-6. Notably, the SASP promotes breast cancer cell proliferation, and simvastatin suppresses this proliferation (Liu et al, 2015).
On the basis of all the results presented in the last two sections (‘bright’ vs ‘dark’ sides), it is increasingly obvious that inhibiting the SASP will need to be judiciously applied in a targeted manner.
Context-dependent effects of senescence and the SASP
Tissue regeneration/differentiation-dependent effects
In addition to genotoxic stress or oncogene activation, mitochondrial dysfunction, mediated by any of several means, results in a distinct senescent phenotype termed mitochondrial dysfunction-associated senescence (MiDAS; Wiley et al, 2016). MiDAS results from a reduced NAD+/NADH ratio, AMPK activation and p53 phosphorylation, which imposes a senescence growth arrest. MiDAS-induced p53 activation suppresses the IL-1 arm of the SASP, leading to a distinct SASP.
In vivo, MiDAS was shown to occur in skin and fat tissues. In culture, the MiDAS SASP inhibits adipogenesis and promotes keratinocyte differentiation (Wiley et al, 2016). At young ages, MiDAS-induced keratinocyte differentiation is beneficial and accelerates wound healing, but is detrimental at old ages where senescence depletes epidermal stem cells and reduces wound healing (Velarde et al, 2015). These studies support the idea that the pleiotropic effects of senescence are inextricably context dependent.
Notably, pyruvate prevents MiDAS in cells with compromised mitochondrial function by restoring cytosolic NAD+/NADH ratios. While these cells are not senescent, they develop a secretory phenotype similar to the SASP observed following genotoxic stress or oncogene activation (Wiley et al, 2016). Much like the pro-inflammatory SASP, these secreted factors promote an epithelia-mesenchymal transition and an invasive phenotype in breast cancer cells, whereas the secretion of cells cultured without pyruvate (MiDAS cells) does not (Wiley and Campisi, unpublished data). Thus, some effects of senescence may depend on the availability of extracellular factors, such as pyruvate, in the context of the local tissue microenvironment.
Effects dependent on senescence dynamics
Mounting evidence supports the idea that the timing of the presence of senescent cells influences the effects they and the SASP have in vivo. For example, developmental senescence was distinctly transient as senescent cells are cleared by immune cells shortly after their appearance. Similarly, during wound healing, senescent cells are present only transiently (Jun and Lau, 2010; Demaria et al, 2014). In contrast, the chronic presence of senescent cells is thought to be responsible for the detrimental effects of senescence (van Deursen, 2014). Ageing of the immune system could further promote the accumulation of senescent cells, thereby reinforcing the deleterious effects of senescence. Thus, the timing of senescence may be as important as the presence of senescent cells in determining whether they have a beneficial or deleterious role in tissue function.
Genetic/metabolic-dependent effects of senescence
The detrimental effects of the SASP may well be due to an increase in the number of senescent cells, which can occur during ageing or in response to environmental stimuli such as chemotherapy or toxic molecules produced by tumour cells. However, the SASP can also change in composition. Epithelial cells induced to senesce by loss of PTEN secrete higher level of the immunosuppressive cytokines CXCL2 and IL-10 than cells that senesce owing to oncogenic mutations in RAS. As a result, more immunosuppressive immune cells infiltrate tumours caused by a loss of PTEN, and consequently these tumours are more invasive than RAS-driven tumours (Toso et al, 2014). It is possible the SASP changes in chronically present senescent cells because these cells experience continuous genetic and epigenetic remodelling (van Deursen, 2014). It is also plausible that metabolic alterations determine the quality of the SASP as the intra- and extra-cellular metabolites can modify the SASP (Quijano et al, 2012; Wiley et al, 2016).
Conclusions
In order to develop more effective anti-cancer therapies, it might be paramount to understand senescent cells in both their beneficial and pathological contexts, particularly because the SASP can also induce paracrine senescence in normal cells, both in culture and in vivo (Acosta et al, 2013). Indeed, as this paracrine-induced secondary senescence also possesses its own, weaker secretory phenotype, these effects could be both tumour-suppressive and tumour-promoting, depending on the context. Although the quality of the SASP can influence cancer progression, modification of the SASP might be a potential therapeutic strategy. Therapies aimed at suppressing the SASP might suppress tumorigenesis (Velarde et al, 2013). Depending on the tumour and tissue context, modulation of the SASP must be controlled in terms of quality and quantity of secreted factors, as well as the timing of secretion. A thorough characterisation of the genetics, epigenetics and metabolism of tumour-promoting senescent cells will be needed to efficiently modify the SASP. If modification is ineffective, killing senescent cells is a potential therapeutic alternative. It is plausible that persistent senescent tumour cells escape immunosurveillance via the cleavage of their antigens. In this situation, it would be efficacious to stimulate other immune cells for which the senescence antigen is still recognisable. If the patient’s immune status is somehow compromised, activating apoptosis can trigger the killing of senescent cells. However, killing senescent cells risks losing their beneficial effects. Thus, although the early preclinical results are promising, senescence-targeted therapies are currently in their infancy. Future studies will determine which therapies are best used to exploit the context-dependent nature of senescent cells.
References
Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15: 978–990.
Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Furnagalli M, DaCosta M, Brown C, Popov N, Takastu, Yabuta N, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, Gil J (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018.
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530: 184–189.
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236.
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120: 513–522.
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D (2015) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22: 78–83.
Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM (2014) Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15: 1139–1153.
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6: 2853–2868.
Davalos AR, Coppe JP, Campisi J, Desprez PY (2010) Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 29: 273–283.
Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31: 722–733.
Freund A, Patil CK, Campisi J (2011) p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J 30: 1536–1548.
Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37: 614–636.
Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH (2013) p53-dependent chemokine production by senescent tumour cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 210: 2057–2069.
Jun JI, Lau LF (2010) The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685.
Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, Iken M, Vucur M, Weiss S, Heikenwalder M, Khan S, Gil J, Bruder D, Manns M, Schirmacher P, Tacke F, Ott M, Luedde T, Longerich T, Kubicka S, Zender L (2011) Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479: 547–551.
Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS (2007) Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest 117: 3421–3426.
Kortlever RM, Higgins PJ, Bernards R (2006) Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 8: 877–884.
Krizhanovsky V, Xue W, Zender L, Yon M, Hernando E, Lowe SW (2008a) Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb Symp Quant Biol 73: 513–522.
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW (2008b) Senescence of activated stellate cells limits liver fibrosis. Cell 134: 657–667.
Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031.
Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9: 81–94.
Laberge RM, Awad P, Campisi J, Desprez PY (2012a) Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron 5: 39–44.
Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez PY, Benz CC, Kapahi P, Nelson PS, Campisi J (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17: 1049–1061.
Laberge RM, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PL, Liu S, Demaria M, Cong YS, Kapahi P, Desprez PY, Hughes RE, Campisi J (2012b) Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell 11: 569–578.
Liu S, Uppal H, Demaria M, Desprez PY, Campisi J, Kapahi P (2015) Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci Rep 5: 17895.
Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, Lowe SW (2013) Non-cell-autonomous tumor suppression by p53. Cell 153: 449–460.
Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M (2013) Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118.
Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J (2009) Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA 106: 17031–17036.
Quijano C, Cao L, Fergusson MM, Romero H, Liu J, Gutkind S, Rovira II, Mohney RP, Karoly ED, Finkel T (2012) Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle 11: 1383–1392.
Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192: 547–556.
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593–602.
Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM (2013) Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155: 1119–1130.
Toso A, Revandkar A, Di Mitri D, Guccini I, Proietti M, Sarti M, Pinton S, Zhang J, Kalathur M, Civenni G, Jarrossay D, Montani E, Marini C, Garcia-Escudero R, Scanziani E, Grassi F, Pandolfi PP, Catapano CV, Alimonti A (2014) Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep 9: 75–89.
van Deursen JM (2014) The role of senescent cells in ageing. Nature 509: 439–446.
Velarde MC, Demaria M, Campisi J (2013) Senescent cells and their secretory phenotype as targets for cancer therapy. Interdiscip Top Gerontol 38: 17–27.
Velarde MC, Demaria M, Melov S, Campisi J (2015) Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells. Proc Natl Acad Sci USA 112: 10407–10412.
Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T (2007) Restoration of p53 function leads to tumour regression in vivo. Nature 445: 661–665.
Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR (2008) Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132: 363–374.
Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J (2016) Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23: 303–314.
Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW (2007) Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445: 656–660.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JC is a founder of Unity Biotechnology, which aims to develop senolytic drugs to treat age-related pathologies. The remaining authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
This work is licensed under the Creative Commons Attribution-Non-Commercial-Share Alike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Lecot, P., Alimirah, F., Desprez, PY. et al. Context-dependent effects of cellular senescence in cancer development. Br J Cancer 114, 1180–1184 (2016). https://doi.org/10.1038/bjc.2016.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.115
Keywords
This article is cited by
-
Mitofusin 1 silencing decreases the senescent associated secretory phenotype, promotes immune cell recruitment and delays melanoma tumor growth after chemotherapy
Scientific Reports (2024)
-
Cancer-associated fibroblasts in radiotherapy: Bystanders or protagonists?
Cell Communication and Signaling (2023)
-
Cellular senescence triggers intracellular acidification and lysosomal pH alkalinized via ATP6AP2 attenuation in breast cancer cells
Communications Biology (2023)
-
Update on the Role of Glucocorticoid Signaling in Osteoblasts and Bone Marrow Adipocytes During Aging
Current Osteoporosis Reports (2023)
-
Exposome and unhealthy aging: environmental drivers from air pollution to occupational exposures
GeroScience (2023)