Abstract
Background:
Gastro-oesophageal adenocarcinomas rarely metastasize to the central nervous system (CNS). The role of the human epidermal growth factor receptor 2 (HER2) in patients with these cancers and CNS involvement is presently unknown.
Patients and Methods:
A multicentre registry was established to collect data from patients with gastro-oesophageal adenocarcinomas and CNS involvement both retrospectively and prospectively. Inclusion in the study required a predefined clinical data set, a central neuro-radiological or histopathological confirmation of metastatic CNS involvement and central assessment of HER2 by immunohistochemistry (IHC) and in situ hybridisation (ISH). In addition, expression of E-cadherin and DNA mismatch repair (MMR) proteins were assessed by IHC.
Results:
One hundred patients fulfilled the inclusion criteria. The population’s median age was 59 years (interquartile range: 54–68), of which 85 (85%) were male. Twenty-five patients were of Asian and 75 of Caucasian origin. HER2 status was positive in 36% (95% CI: 26.6–46.2) of cases. Median time from initial diagnosis to the development of brain metastases (BMets) or leptomeningeal carcinomatosis (LC) was 9.9 months (95% CI: 8.5–15.0). Median overall survival from diagnosis was 16.9 months (95% CI: 14.0–20.7) and was not related to the HER2 status. E-cadherin loss was observed in 9% of cases and loss of expression in at least one DNA MMR proteins in 6%.
Conclusions:
The proportion of a positive HER2 status in patients with gastro-oesophageal adenocarcinoma and CNS involvement was higher than expected. The impact of anti-HER2 therapies should be studied prospectively.
Similar content being viewed by others
Main
Adenocarcinoma of the stomach, including the gastro-oesophageal junction, is the fifth most common cancer in the world, and is predominantly diagnosed in Eastern Asia. Peak onset of the disease is between 60 and 80 years of age and age-standardised incidence rates of gastro-oesophageal adenocarcinoma are twice as high in males than in females. There is significant geographic variation with age-standardised incidence rates ranging from 3.3 in Western Africa to 35.4 in Eastern Asia for men, and from 2.6 in Western Africa to 13.8 in Eastern Asia for women (http://ci5.iarc.fr). A better clinical outcome has been reported for Asians, females and young age (Al-Refaie et al, 2008; Kunz et al, 2012).
Metastases from gastro-oesophageal adenocarcinoma primarily involve the peritoneal cavity, liver and lymph nodes (Maehara et al, 2000; D’Angelica et al, 2004). Metastases to the brain are rare (York et al, 1999; Go et al, 2011) and occur in less than 5% of patients with adenocarcinoma of the upper gastrointestinal tract (Wadhwa et al, 2013; Blay et al, 2015).
The product of the HER2 gene, a member of the epidermal growth factor (EGF) receptor family, is overexpressed and the gene is amplified in 10–22% of patients with gastro-oesophageal adenocarcinoma, both in Asian and in Caucasian patients. The variability in HER2 positivity reported in different studies can partially be explained by intratumoural heterogeneity, insufficient tissue sampling or inconsistent interpretation criteria (Gravalos and Jimeno, 2008; Hofmann et al, 2008; Bang et al, 2010; Grabsch et al, 2010; Janjigian et al, 2012; Warneke et al, 2013a, 2013b; Van Cutsem et al, 2014).
In gastric cancer, the ToGa trial has recently established HER2 as a predictive marker for response to the monoclonal anti-HER2 antibody trastuzumab. This trial did not include patients with brain metastases (BMets) (Bang et al, 2010).
Few studies have analysed the clinical significance of HER2 in patients with gastro-oesophageal adenocarcinoma and CNS involvement. In a retrospective cohort of 140 patients with oesophageal cancer, 9 patients developed BMets—5 of them were HER2 positive based on immunohistochemistry (IHC) (Abu Hejleh et al, 2012). In a small series of 20 patients with BMets of gastro-oesophageal adenocarcinoma, three were HER2 positive based on IHC and fluorescence in situ hybridisation (FISH) analysis (Preusser et al, 2013). Since patients with HER2 positive breast cancer were reported to develop BMets more frequently and more rapidly than patients with HER2 negative cancers (Pestalozzi et al, 2006; Musolino et al, 2011), we aimed to establish the frequency of HER2 positivity in patients with brain metastases from gastro-oesophageal adenocarcinoma.
E-cadherin (encoded by the CDH1 gene), a transmembrane glycoprotein involved in maintaining cell–cell contact, has recently been implicated in mediating BMets. Studies examining BMets tissue from lung cancer (Kafka et al, 2014) have reported a frequent loss of E-cadherin expression, suggesting a potential role in BMets development. Since both, loss of E-cadherin expression and mutation (deletion), have been associated with worse prognosis in gastric cancer (Uchikado et al, 2011; Corso et al, 2013), we also wanted to investigate the relationship between E-cadherin status and its relationship with clinicopathological variables in our study population.
Microsatellite instability (MSI), an alteration caused by genetic and epigenetic inactivation of DNA mismatch repair (MMR) genes is usually related to a loss of function of the MMR enzyme system. In sporadic gastric cancer, MMR deficiency occurs in about 10% of cases and has been linked to a more favourable clinical course (Lee et al, 2002; Warneke et al, 2013a, 2013b; Choi et al, 2014). We therefore anticipated MMR deficiency to be a rare event in our study population.
To investigate the status of HER2, E-cadherin, and MSI we established a registry of European and Asian patients with gastro-oesophageal adenocarcinomas who had developed metastases to the brain and/or to the leptomeninges.
Patients and methods
Eligible patients met the following criteria: Histologically confirmed adenocarcinoma of the stomach or oesophagus; availability of a clinical data set as defined in the study protocol; central nervous system (CNS) involvement confirmed by central neuro-radiological or histopathological review (cytology, surgical pathology, or autopsy); in the absence of brain images, the medical reports had to demonstrate conclusive evidence of BMets; availability of tumour tissue (from primary and/or from CNS or visceral metastases) for histological review, assessment of HER2 status, expression of E-cadherin and DNA MMR proteins.
The ethics committee of the Canton of Zurich, Switzerland (KEK-ZH-NR: 201 0-0445) and the ethics committees of all collaborating centres approved the study, which is registered on www.ClinicalTrials.gov, NCT01456455.
The local investigators collected clinical and follow-up data from medical records either from hospital records or from the patients’ general practitioners. Written informed consent was obtained from all patients alive at the time of their inclusion into the study.
Neuroradiology
The review was performed by a board certified neuroradiologist specialised in neuro-oncology (BS). The patient’s neuro-imaging studies, including MRI and CT scans, were analysed on a state of the art PACS (Picture and Archiving Communication System) and categorized according to the presence of CNS metastases as a single lesion, multiple lesions (⩽3 or >3), and/or as leptomeningeal carcinomatosis (LC). Metastases to the CNS were defined as one or multiple contrast enhancing lesions in the brain, in the lepto- or pachymeninges, or in the ependymal lining of the ventricular system. MRIs were analysed based on standardized sequences that is, axial T1, T2, and FLAIR-weighted series with a slice thickness of 4 mm and 3D contrast-enhanced sequence with 1 mm slice thickness reconstructed in three planes. CT scans included non-contrast and contrast exams with 1 mm slice thickness and with reconstruction of the contrast-enhanced series performed in three planes.
Histopathological assessment
Paraffin blocks and/or unstained slides with sufficient tumour tissue were submitted for central pathology review to the Institute of Surgical Pathology, University Hospital Zurich, Switzerland, where the pathological diagnosis (e.g., the presence of adenocarcinoma) was confirmed either based on the review of glass slides or based on the review of digital slides by the study’s principal pathologist (ZV).
Histological subtypes and grade of differentiation were determined according to the Laurén (1965) and the WHO classification (Bosman et al, 2010). Adenocarcinomas were categorised as intestinal, diffuse, or mixed type. Adenocarcinomas with signet-ring cells were classified as diffuse type. All diffuse type cancers were graded as poorly differentiated (G3), whereas intestinal type cancers were graded as well (G1), moderate (G2), or poorly (G3) differentiated.
HER2 immunohistochemistry
HER2 protein was assessed using the FDA-approved ready-to-use antibody, PATHWAY anti-HER2/neu clone 4B5 (Ventana Medical Systems, Basel, Switzerland). The immunohistochemical procedure was carried out using the Leica Bond autostainer (Leica Biosystems, Nunningen, Switzerland) or the Ventana Bench Mark Ultra autostainer.
Scoring was performed in accordance with the guidelines of the ToGA trial (Bang et al, 2010) and the corresponding literature (Rüschoff et al, 2012).
HER2 fluorescence or silver enhanced in situ hybridisation
All cases tested in Zurich, except for patient 100, and all cases from the Netherlands Cancer Institute (NCI), patients 87–99, were tested using silver enhanced in situ hybridisation (SISH). Patient 100 was analysed using FISH.
SISH HER2 analysis
HER2 copy number status determined with SISH was performed using a dual probe (INFORM HER2 Dual ISH assay, Ventana Medical Systems, Tucson, AZ, USA, catalog Nr: 780-4332) containing the HER2 region (17q11.2–q12, directly labelled with black) and the centromeric region of chromosome 17 (17p11.1–q11.1, directly labelled with red). The signals were detected with the UltraView SISH detection kit and the UltraView red ISH detection kit as per instructions by the manufacturer. The in situ hybridisation procedure was carried out using the Ventana Benchmark autostainer according to the manufacturer recommendation.
At the NCI, ISH Protease 3 (cat. #5273331001, Ventana Medical Systems), INFORM HER2 DNA Probe (cat. #5273439001, Ventana Medical Systems), and Rabbit anti-DNP (cat. #5273447001, Ventana Medical Systems) were used. Bound probe was visualised using silver deposition (ultraVIEW SISH Detection Kit, Ventana Medical Systems) followed by haematoxylin counterstain.
Scoring of SISH signals followed the guidelines established in the ToGA trial (Bang et al, 2010).
FISH HER2 analysis (case 100)
Fluorescence in situ hybridisation was used to establish the HER2 copy number status in case 100 as described previously (Varga et al, 2012). The FISH scoring was performed in analogy to the scoring applied for SISH.
In summary, HER2 positive tumours were defined as IHC score 3+ or ISH amplified (FISH or SISH) in at least one probe (primary tumour and/or metastastic lesion).
E-cadherin immunohistochemistry
E-cadherin IHC was performed using either clone EP700Y (Cell Marque, dilution 1 : 200) and the Ventana Benchmark autostainer or clone NCH-38 (DAKO; Cat. #M3612, dilution 1 : 50) followed by the UltraView Universal DAB Detection Kit (Ventana Medical Systems).
Any membranous immunoreactivity, irrespective of the number of cells or the intensity, was scored as positive. The absence of membranous immunoreactivity or the presence of cytoplasmic stain only was scored as negative.
DNA MMR proteins IHC
Immunohistochemistry for MSH2 (clone 25D12, Novocastra (Biosystems Switzerland, Muttenz, Switzerland), dilution 1 : 100; or Clone G219-1129, Roche Ventana Medical Systems, ready-to-use), MSH-6 (clone 44, Becton Dickinson, Allschwil, Switzerland, dilution 1 : 500; or Clone EP49, Epitomics (LabForce, Nunningen, Switzerland), dilution 1 : 50), MLH1 (clone G168-15, Becton Dickinson, dilution 1 : 100; or clone M1, Roche Ventana Medical Systems, ready-to-use) and PMS2 (clone A16-4, Becton Dickinson, dilution 1 : 300; or Clone EPR3947, Roche Ventana Medical Systems, ready-to-use) was performed using the Ventana Benchmark system or using the OptiView DAB Detection Kit (Ventana Medical Systems) followed by counterstaining with haematoxylin.
Any nuclear staining in the tumour cells was considered as ‘positive’. Complete absence of immunoreactivity in the presence of a positive internal control (lymphocytes) was scored as ‘negative’.
Statistics
Descriptive statistics were calculated for the variables of interest, and stratified by subgroups of HER2 status (positive vs negative) and ethnicity (Asian vs Caucasian). Overall survival was calculated from initial diagnosis to death or last follow-up. Survival from BMets was calculated from diagnosis of BMets to death or last follow-up. Time to BMets was calculated from initial diagnosis to the diagnosis of BMets. We obtained median survival times and their 95% confidence intervals using the Kaplan–Meier method. Results are presented for all patients as well as for the subgroups by HER2 status. All analyses were conducted with R (R Core Team, 2015). A P-value of 0.05 was set as statistically significant.
Results
Patients
A total of 129 patients were included in the registry from 2011 to 2013. Of these, 100 fulfilled the inclusion criteria for this study (see Appendix).
Twenty-nine registered patients were excluded because (i) there was no tissue available for confirmation of the diagnosis and further analysis (8), (ii) squamous cell histology (7), (iii) lack of clinical data (11), or (iv) the absence of radiologically or histologically confirmed BMets or LC (3) (Figure 1).
The median age of the included study patients was 59 years (interquartile range 54–68 years) and 85 (85%) were males, 25 (25%) were of Asian ethnicity and 75 (75%) were Caucasian. Forty-five (45%) tumours were located at the cardia and 53 (53%) were classified as ‘non-cardia’ cancers, of which 32 (32%) were located in the lower oesophagus (in two cases the tumour localisation was unknown). In 21 (21%) patients BMets were diagnosed synchronously and in 79 (79%) metachronously.
CNS involvement
Central nervous system involvement was confirmed in 90 patients by diagnostic imaging or by CNS histology or cytology. For 10 patients, MRI or CT scans were not available for review, however, the provided patient medical reports demonstrated conclusive evidence of BMets. Three patients had to be excluded from further analysis due to diagnostic errors (cerebral embolic infarction in two patients and cerebral hemorrhage in one).
CNS involvement was as follows: BMets (n=74, 74%), LC alone (n=15, 15%), BMets and LC (n=11, 11%). Extracerebral metastases were present in 80% of patients (in 6% no information was available). Four of the thirty-six patients with HER2 positive tumours received trastuzumab for metastatic disease. Patient characteristics are summarised in Table 1.
Histopathological review
Tissue was available from the primary tumour in 75% of all patients; in 13% of the cases, primary tumour tissue and matched tissue from at least one distant metastasis could be analysed (eight metastases from the brain and five metastases from lung, lymph nodes and/or liver). In 23 cases, tissue from one or more metastatic sites was available and in two tumour samples (2%) no precise anatomical location was possible.
Sixty percent of the cancers were intestinal-type, 24% diffuse-type, and 12% were classified as mixed-type. Four percent were adenosquamous carcinomas. Twenty-five percent were moderately differentiated (G2) and 75% were poorly differentiated (G3) (Table 2).
HER2 status
Thirty-six (36%) of the cancers were HER2 positive either in the primary tumour or in a metastatic lesion. In 11 cases, the HER2 status was concordant between the primary tumour and matched metastases. In two cases, the primary tumour was HER2 negative and the matched visceral metastases were HER2 positive.
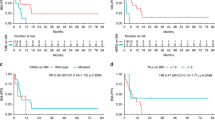
The median time from initial diagnosis to the development of metachronous BMets or LC was 9.9 months (95% CI: 8.5–15.1). Patients with HER2 positive primaries developed BMets/LC within a shorter time than patients with HER2 negative primaries (8.9 months, 95% CI: 4.3–14.7 vs 12.7 months, 95% CI: 8.8–17.2, log-rank P=0.157, not significant). The median overall survival of all patients was 16.9 months (95% CI: 14.4–20.7); there was no significant difference in survival between patients with HER2 positive tumours (17.4 months, 95% CI: 12.1–26.2) and those with HER2 negative tumours (16.4 months, 95% CI: 14.1–21.0). Median survival for patients with synchronous BMets (n=21) was 5.2 months (95% CI: 3.5–16.9) whereas for metachronous BMets (n=79) median survival reached 19.0 months (95% CI: 16.4–26.2). The median survival from diagnosis of CNS involvement of all patients was 3.4 months (95% CI: 2.3–4.7); there was no significant difference in survival between patients with HER2 positive tumours (4.2 months, 95% CI 3.4–7.4) and HER2 negative tumours (2.3 months, 95% CI 1.8–4.7) (Table 3).
E-cadherin status
E-cadherin data were available for 98 (98%) cases. Nine (9%) demonstrated a complete loss of E-cadherin immunostaining. There was no discrepancy between matched tissue pairs. Outcome between E-cadherin positive and E-cadherin negative patients was identical.
Expression of DNA MMR proteins
Expression of DNA MMR proteins (MLH1, MSH2, MSH6, and PMS2) was assessed in 99 of 100 cases. Loss of expression of at least one of the proteins was seen in 6%. MLH1 and PMS2 were absent in four cases. MLH1, PMS2, and MSH6 were absent in two cases. Loss of MSH2 was not observed. One of 12 matched tissue pairs showed loss of MLH1 and PMS2 in the cerebral metastasis but not in the corresponding extracerebral tumour, all other pairs showed concordance. There was no difference in outcome between MMR proficient and MMR deficient patients.
Discussion
In the present study, we investigated the frequency of HER2 positivity, E-cadherin status, and DNA MMR protein expression and their relationship with patient outcome in a large cohort of patients with CNS metastases of gastro-oesophageal adenocarcinomas. Brain metastases from upper GI cancer are very rare; therefore, the number of patients collected within our registry is substantial and unlikely to be reproduced in a prospective trial.
In our study cohort of 100 patients, 36% had a HER2 positive tumour. This incidence is higher than the reported proportion of HER2 positivity in gastro-oesophageal adenocarcinoma (Gravalos and Jimeno, 2008; Hofmann et al, 2008; Bang et al, 2010; Grabsch et al, 2010; Janjigian et al, 2012; Warneke et al, 2013a, 2013b; Van Cutsem et al, 2014). Whether our findings suggest a propensity to develop CNS metastases in this patient subgroup or rather reflects a group of patients who lived long enough to develop BMets remains to be investigated.
In cases where tissue was available from the primary tumour and matched metastases (n=13), the HER2 status was concordant except for two cases, which could reflect spatial intratumour heterogeneity (Bozzetti et al, 2011; Shibata et al, 2014).
Twenty-six percent of our patients had leptomeningeal carcinomatosis (LC), a disease manifestation that is even rarer than brain metastases. In a large series of gastric adenocarcinoma, LC occurred in 8 of 5618 patients with gastric (0.15%) and 7 of 4361 (0.16%) with oesophageal adenocarcinoma (Giglio et al, 2005). In our cohort, LC was not related to HER2 status.
Median survival of all patients was longer than expected for patients with advanced disease. While we did not see any relationship between HER2 status and survival, our cohort of virtually trastuzumab-naïve patients with HER2 positive cancers seems to develop BMets more rapidly than patients with HER2 negative cancers, although the difference was not statistically significant. Similar findings have been reported for breast cancer (Musolino et al, 2011). Given the limited number of patients, our observation will need to be confirmed.
The expression of E-cadherin and DNA MMR proteins was similar between primary tumours and CNS metastases and did not influence the clinical course of the disease.
Even though this is the largest cohort reported to date, 100 evaluable patients with gastro-oesophageal adenocarcinomas and CNS involvement are still a relatively small number. A substantial part of the analyses was performed retrospectively and may thus be subject to bias. Some clinical data such as performance status, a major prognostic factor for patients with brain metastases (Sperduto et al, 2012), were not available for the retrospective part of the study. Furthermore there is no matched control group without BMets, thus we had to compare our results with data from the literature.
All the same, our collaborative study has several important strengths: centralised HER2 status assessment, central histopathology review, and central review of clinical data reduced technical sources of variation. Centralised assessment of the cranial imaging increased the likelihood that CNS involvement was accurately diagnosed. Finally, representation of Caucasian and Asian patients enhances the generalisability of our study findings.
In conclusion, we could show that HER2 is frequently overexpressed and amplified in patients with gastro-oesophageal adenocarcinomas and CNS involvement. HER2-targeted therapies may favourably alter the prognosis of these patients and may prolong the time to involvement of the brain and the meninges.
Change history
01 September 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abu Hejleh T, DeYoung BR, Engelman E, Deutsch JM, Zimmerman B, Halfdanarson TR, Berg DJ, Parekh KR, Lynch WR, Iannettoni MD, Bhatia S, Clamon G (2012) Relationship between HER-2 overexpression and brain metastasis in esophageal cancer patients. World J Gastrointest Oncol 4 (5): 103–108.
Al-Refaie WB, Tseng JF, Gay G, Patel-Parekh L, Mansfield PF, Pisters PW, Yao JC, Feig BW (2008) The impact of ethnicity on the presentation and prognosis of patients with gastric adenocarcinoma. Results from the National Cancer Database. Cancer 113 (3): 461–469.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet 376 (9742): 687–697.
Blay C, Chiforeanu DC, Boucher E, Cabillic F, Desgrippes R, Leconte B, Perrin C, Manfredi S, Audrain O, Meunier B, Edeline J (2015) Incidence of brain metastases in HER2+ gastric or gastroesophageal junction adenocarcinoma. Acta Oncol 1–3.
Bosman FT, Carneiro F, Hruban RH, Theise RH (ed) (2010) WHO Classification of Tumours of the Digestive System 4th edn Vol. 3. . IARC Press: Lyon.
Bozzetti C, Negri FV, Lagrasta CA, Crafa P, Bassano C, Tamagnini I, Gardini G, Nizzoli R, Leonardi F, Gasparro D, Camisa R, Cavalli S, Silini EM, Ardizzoni A (2011) Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer 104 (9): 1372–1376.
Choi YY, Bae JM, An JY, Kwon IG, Cho I, Shin HB, Eiji T, Aburahmah M, Kim HI, Cheong JH, Hyung WJ, Noh SH (2014) Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J Surg Oncol 110 (2): 129–135.
CI5 (2015) Cancer Incidence in Five Continents (Electronic Version). IARC: Lyon, Available at http://ci5.iarc.fr . (last accessed 18 April 2015).
Corso G, Carvalho J, Marrelli D, Vindigni C, Carvalho B, Seruca R, Roviello F, Oliveira C (2013) Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J Clin Oncol 31 (7): 868–875.
D'Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS (2004) Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 240 (5): 808–816.
Giglio P, Weinberg JS, Forman AD, Wolff R, Groves MD (2005) Neoplastic meningitis in patients with adenocarcinoma of the gastrointestinal tract. Cancer 103 (11): 2355–2362.
Go PH, Klaassen Z, Meadows MC, Chamberlain RS (2011) Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer 117 (16): 3630–3640.
Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W (2010) HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell Oncol 32 (1-2): 57–65.
Gravalos C, Jimeno A (2008) HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 19 (9): 1523–1529.
Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T (2008) Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52 (7): 797–805.
Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, Altmannsberger HM, Robinson E, Tafe LJ, Tang LH, Shah MA, Al-Batran SE (2012) Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol 23 (10): 2656–2662.
Kafka A, Tomas D, Beroš V, Pećina HI, Zeljko M, Pećina-Šlaus N (2014) Brain metastases from lung cancer show increased expression of DVL1, DVL3 and beta-catenin and down-regulation of E-cadherin. Int J Mol Sci 15 (6): 10635–10651.
Kunz PL, Gubens M, Fisher GA, Ford JM, Lichtensztajn DY, Clarke CA (2012) Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol 30 (28): 3507–3515.
Laurén P (1965) The two histological main types of gastric carcinoma: diffuse and so –called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64: 31–49.
Lee HS, Choi SI, Lee HK, Kim HS, Yang HK, Kang GH, Kim YI, Lee BL, Kim WH (2002) Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol 15 (6): 632–640.
Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K (2000) Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg 87 (3): 353–577.
Musolino A, Ciccolallo L, Panebianco M, Fontana E, Zanoni D, Bozzetti C, Michiara M, Silini EM, Ardizzoni A (2011) Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer 117 (9): 1837–1846.
Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, Crivellari D, Fey MF, Murray E, Pagani O, Simoncini E, Castiglione-Gertsch M, Gelber RD, Coates AS, Goldhirsch A International Breast Cancer Study Group (IBCSG) (2006) International Breast Cancer Study Group (IBCSG). Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 17 (6): 935–944.
Preusser M, Berghoff AS, Ilhan-Mutlu A, Dinhof C, Magerle M, Marosi C, Hejna M, Capper D, von Deimling A, Schoppmann SF, Birner P (2013) Brain metastases of gastro-oesophageal cancer: evaluation of molecules with relevance for targeted therapies. Anticancer Res 33 (3): 1065–1071.
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL. http://www.R-project.org/.
Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G (2012) HER2 testing in gastric cancer: a practical approach. Mod Pathol 25 (5): 637–650.
Shibata R, Nimura S, Hashimoto T, Miyake T, Takeno S, Hoshino S, Nabeshima K, Yamashita Y (2014) Expression of human epidermal growth factor receptor 2 in primary and paired parenchymal recurrent and/or metastatic sites of gastric cancer. Mol Clin Oncol 2 (5): 751–755.
Sperduto PW, Kased N, Roberge D (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. Surveillance. J Clin Oncol 30 (4): 419–425.
Uchikado Y, Okumura H, Ishigami S, Setoyama T, Matsumoto M, Owaki T, Kita Y, Natsugoe S (2011) Increased Slug and decreased E-cadherin expression is related to poor prognosis in patients with gastric cancer. Gastric Cancer 14 (1): 41–49.
Van Cutsem E, Bang YJ, Feng-Yi F, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J (2014) HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18 (3): 476–484.
Varga Z, Tubbs RR, Wang Z, Sun Y, Noske A, Kradolfer D, Bosshard G, Jochum W, Moch H, Öhlschlegel C (2012) Co-amplification of the HER2 gene and chromosome 17 centromere: a potential diagnostic pitfall in HER2 testing in breast cancer. Breast Cancer Res Treat 132 (3): 925–935.
Wadhwa R, Taketa T, Correa AM, Sudo K, Campagna MC, Blum MA, Komaki R, Skinner H, Lee JH, Bhutani MS, Weston B, Maru DM, Rice DC, Swisher S, Hofstetter WL, Ajani JA (2013) Incidence of brain metastases after trimodality therapy in patients with esophageal or gastroesophageal cancer: implications for screening and surveillance. Oncology 85 (4): 204–207.
Warneke VS, Behrens HM, Böger C, Becker T, Lordick F, Ebert MP, Röcken C (2013a) Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann Oncol 24 (3): 725–733.
Warneke VS, Behrens HM, Haag J, Balschun K, Böger C, Becker T, Ebert MP, Lordick F, Röcken C (2013b) Prognostic and putative predictive biomarkers of gastric cancer for personalized medicine. Diagn Mol Pathol 22: 127–137.
York JE, Stringer J, Ajani JA, Wildrick DM, Gokaslan ZL (1999) Gastric cancer and metastasis to the brain. Ann Surg Oncol 6 (8): 771–776.
Acknowledgements
We would like to acknowledge the Netherlands Cancer Institute Core Facility Molecular Pathology and Biobanking (CFMPM) for support. This work was supported in part by an unrestricted grant from ROCHE Pharma, Switzerland (SH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in part at the 2nd EORTC St Gallen Gastrointestinal Cancer Congress, 6–8 March 2014, the DGHO Annual Meeting Hamburg, 10–14 October 2014, and the UAE Cancer Congress, Dubai, 30 October 30–1 November 2014.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Appendix
Appendix
The following cancer centres contributed to the study: The Department of Oncology and Institute of Surgical Pathology, University Hospital Zurich, Switzerland (n=12); Pathology Section, National Cancer Centre, Tokyo, Japan (n=25); The Department of Oncology and Institute of Pathology, Cantonal Hospital St Gallen, Switzerland (n=26); The Department of Radiation Oncology, Cantonal Hospital of Valais, Sion, Switzerland (n=10); The Department of Radiation Oncology and Institute of Pathology, Cantonal Hospital Luzern, Switzerland (n=8); The Netherlands Cancer Institute, The Netherlands (n=13); and three further centres in Switzerland (n= 6).
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Feilchenfeldt, J., Varga, Z., Siano, M. et al. Brain metastases in gastro-oesophageal adenocarcinoma: insights into the role of the human epidermal growth factor receptor 2 (HER2). Br J Cancer 113, 716–721 (2015). https://doi.org/10.1038/bjc.2015.279
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.279
Keywords
This article is cited by
-
Leptomeningeal carcinomatosis and brain metastases in gastroesophageal carcinoma: a real-world analysis of clinical and pathologic characteristics and outcomes
Journal of Neuro-Oncology (2024)
-
Early detection of brain metastases and appropriate local therapy followed by systemic chemotherapy may improve the prognosis of gastric cancer
Scientific Reports (2023)
-
The relationship between brain metastasis and HER2 expression status in gastric cancer
Clinical and Translational Oncology (2023)
-
Erythroblastic oncogene B-2 status and intracranial metastatic disease in patients with gastrointestinal cancer: a systematic review
Journal of Neuro-Oncology (2022)
-
The risk and prognostic factors for brain metastases in esophageal cancer patients: an analysis of the SEER database
BMC Cancer (2021)