Abstract
Background:
Peripheral blood-derived inflammation-based scores such as the neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) have recently been proposed as prognostic markers in solid tumours. Although evidence to support these markers as unfavourable prognostic factors is more compelling in gastrointestinal cancers, very little is known of their impact on breast cancer. We investigated the association between the NLR and PLR, and overall survival after breast cancer.
Methods:
Data from the University of Malaya Medical Centre Breast Cancer Registry was used. Of 2059 consecutive patients diagnosed from 2000 to 2008, we included 1435 patients with an available pre-treatment differential blood count (∼70%). Patients were stratified into quintiles of the NLR/PLR. Multivariable Cox regression was used to determine the independent prognostic significances of the NLR/PLR.
Results:
Compared with the first quintile of the NLR, women in quintile 5 were younger, had bigger tumours, nodal involvement, distant metastases and higher tumour grades. Higher NLR quintiles were significantly associated with poorer survival with a 5-year relative survival ratio (RSR) of 76.4% (95% CI: 69.6–82.1%) in quintile 1, 79.4% (95% CI: 74.4–83.7%) in quintile 2, 72.1% (95% CI: 66.3–77.3%) in quintile 3, 65.6% (95% CI: 59.8–70.8%) in quintile 4 and 51.1% (95% CI: 43.3–58.5%) in quintile 5. Following adjustment for demography, tumour characteristics, treatment and the PLR, the adjusted hazard ratio (HR) for quintile 5 vs quintile 1 was 1.50 (95% CI: 1.08–1.63); Ptrend=0.004. Results were unchanged when the NLR was analysed as a dichotomous variable using different cutoff points. Although patients in PLR quintile 5 had lower survival than in quintile 1 (5-year RSR: 53.2% (95% CI: 46.9–59.2%) vs 77.0% (95% CI: 70.9–82.2%)), this association was not significant after multivariable adjustment. However, a PLR >185 was significantly associated with poorer survival; adjusted HR: 1.25 (95% CI: 1.04–1.52).
Conclusions:
Both the NLR and PLR are independently associated with an increased risk of mortality in breast cancer. Their added value in the prognostication of breast cancer in clinical practice warrants investigation.
Similar content being viewed by others
Main
Cancer progression and prognosis are affected by the host’s inflammatory response in the tumour microenvironment (Hanahan and Weinberg, 2011). As components of systemic inflammatory response, lymphocytes, neutrophils, and platelets are increasingly being recognised to have an important role in carcinogenesis and tumour progression (DeNardo and Coussens, 2007; Gregory and Houghton, 2011; Lal et al, 2013). To date, a number of peripheral blood-derived inflammation-based scores such as the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), and Glasgow Prognostic Score have been proposed as prognostic markers in cancer (Roxburgh and McMillan, 2010; Proctor et al, 2011; Guthrie et al, 2013).
Evidence to support NLR as an unfavourable prognostic factor is most compelling in colorectal cancer (Li et al, 2014). Likewise, an elevated PLR has been found to adversely impact survival in gastrointestinal cancers (Templeton et al, 2014a). However, the role of these biomarkers in breast cancer prognosis is less well known (Azab et al, 2012, 2013; Noh et al, 2013; Dirican et al, 2014; Krenn-Pilko et al, 2014; Nakano et al, 2014). To date, several studies have shown that an increased NLR is associated with lower survival (Azab et al, 2012, 2013; Noh et al, 2013; Dirican et al, 2014; Nakano et al, 2014), whereas one study has shown that the PLR may also be an adverse prognostic marker in breast cancer (Krenn-Pilko et al, 2014).
The NLR and PLR can be derived from the full blood count, and may therefore provide a simpler and cheaper avenue for breast cancer prognostication. Validating the findings of previous studies within a large prospective cohort of breast cancer patients in a different setting will hence be useful in elucidating the prognostic role of the NLR and PLR in women with breast cancer. We investigated the association between the pre-treatment NLR and PLR and survival following breast cancer in a large Asian cohort.
Patients and Methods
Data for this study were obtained from the Breast Cancer Registry of the University of Malaya Medical Centre (UMMC), Malaysia. Prospective registration of newly diagnosed breast cancer patients began at the UMMC in 1993. The Registry, which obtained ethical approval from the Institutional Review Board, currently retains detailed data on patients’ demographic, tumour and treatment characteristics (Pathy et al, 2011).
In the year 2000, a fully computerised online laboratory system was introduced at the UMMC, which allows for the tracing of patients’ blood count results. Hence, we included women diagnosed from the year 2000 onwards. Only pre-operative blood count results (counts taken as part of pre-operative assessment) or counts taken before systemic therapy/radiotherapy in those who did not undergo surgery were considered in this study. Of the 2059 consecutive patients diagnosed between 1 January 2000 and 31 December 2008, we have included 1435 patients with available data for pre-treatment differential blood counts (∼70%).
Study variables
In order to ascertain that the blood count results that were extracted from the hospital’s online records were pre-treatment values, we crosschecked the dates of the initial treatment initiation for each patient, against the date when the complete blood count was performed. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. The PLR was defined as the absolute platelet count divided by the absolute lymphocyte count.
The data on the patient’s demography included age at diagnosis, and self-reported ethnicity (Malay, Chinese, Indian, or other race). The variables for the tumour characteristics included pathologically determined tumour size (cm), the number of histologically positive lymph nodes, oestrogen receptor (ER)/progesterone receptor (PR) status (positive when >10% of tumour cells stained positive during immunohistochemical (IHC) testing, negative otherwise), tumour grade (Scarff—Bloom—Richardson classification; grade 1, grade 2, grade 3), lymphovascular invasion (LVI) (present, absent), and HER2 status (positive when IHC testing=3+, negative when IHC testing=0 or 1+). Tumours with equivocal HER2 status (2+) were subjected to fluorescence in situ hybridisation to confirm HER2 positivity. All patients diagnosed prior to January 2003 were restaged using AJCC6 criteria. In 164 patients whom did not undergo surgery, clinically determined tumour size, and axillary lymph node status were used.
Loco-regional treatment data included surgery (no surgery, mastectomy, breast-conserving surgery, adjuvant radiotherapy (yes, no), and surgical margin status (positive, negative)). Systemic treatment data comprised of neoadjuvant chemotherapy (yes, no), adjuvant chemotherapy (no chemotherapy, yes: first generation, yes: second generation (anthracycline based), yes: third generation (taxane based)), and endocrine therapy (yes, no).
Follow-up and outcome assessment
Patients were followed-up in the breast clinic. Vital status was determined through direct linkage with the Malaysian National Registration Department. The follow-up time was calculated from the date of diagnosis to the date of death, or was censored at the end of follow-up (1 February 2014). The cause of death and the data on disease recurrence were only sparsely available.
Statistical analysis
Categorical variables were compared using the χ2-test. Continuous variables were expressed in medians and were compared using the Kruskal–Wallis test. Patients were stratified into the quintiles of the NLR/PLR (quintile 1, quintile 2, quintile 3, quintile 4, quintile 5). The demographic, tumour, and treatment characteristics were compared between the quintiles. Multivariable logistic regression was performed to identify factors that were independently associated with an elevated NLR/PLR. For this purpose, elevations of the NLR and PLR were defined using cutoff values of 4.0 (Dirican et al, 2014; Templeton et al, 2014b) and 185 (Azab et al, 2013; Templeton et al, 2014a), respectively.
To approximate disease-specific survival, we computed relative survival, which is a widely employed measure of cancer survival, given that it does not rely on an accurate cause of death coding (Dickman et al, 2004; Coleman et al, 2008). The relative survival ratio (RSR) is the ratio of overall (all-cause) survival observed in breast cancer patients to the survival that would have been expected had they been subjected only to the background mortality rates of the general female population (matched for age, and calendar year). Expected survival was derived from the Malaysian life tables. The RSR between the five quintiles of the NLR/PLR were compared.
We used multivariable Cox regression analysis to estimate the relative risk of all-cause mortality in each quintile of the NLR/PLR with quintile 1 as the reference, adjusted for all of the previously mentioned variables. Given that both the NLR and PLR are positively associated with increased risk of cancer mortality (Templeton et al, 2014a, 2014b), we mutually adjusted the NLR and PLR against each other.
Previous studies, which investigated the prognostic role of the NLR/PLR have used different cutoff points to define the NLR/PLR elevation. We performed sensitivity analyses using these different cutoff values; NLR: 3.00 (Templeton et al, 2014b), 4.00 (Dirican et al, 2014; Templeton et al, 2014b) and PLR: 185 (Azab et al, 2013), 292 (Krenn-Pilko et al, 2014).
As some studies have shown that the NLR and PLR may only be of prognostic value in certain breast cancer subtypes, we assessed for effect modification (Noh et al, 2013; Krenn-Pilko et al, 2014). Patients were classified as having one of four subtypes of breast cancer; ER-positive or PR-positive and HER2-negative tumours (ER+/PR+ and HER−), ER-positive or PR-positive and HER2-positive tumours (ER+/PR+ and HER2+), ER-negative and PR-negative and HER2-positive tumours (ER− and PR− and HER2+), and ER-negative and PR-negative and HER2-negative tumours (ER− and PR− and HER2−). We performed a log likelihood ratio test by including an interaction term ‘breast cancer subtype (four groups) multiplied by the NLR/PLR (in two categories using cutoff values of 4.0 and 185, respectively)’ into the main Cox model. As most of the subgroups were small, only the prognostic factors that changed the hazard ratio (HR) for an elevated NLR/PLR by >10% in the bivariable analyses were included in the multivariable model.
Missing values (ranging between 5 and 30%) were imputed by multiple imputation. All of the variables in the multivariable Cox regression were included in the imputation model and 10 imputation sets were created.
This study was approved and received ethical clearance from the Medical Ethics Committee of the UMMC (Inst/IRB/1024.73).
Results
The median age at diagnosis was 52 years. A majority of patients were Chinese (58%), followed by Malays (25%), Indians (16%), and other races (1%). The median tumour size at diagnosis was 3.5 cm. Approximately half of the patients had lymph node involvement. At initial diagnosis, about 22% of women presented with (AJCC6) stage I, followed by 34% with stage II, 30% with stage III, and 14% with stage IV breast cancer.
The median pre-treatment NLR and PLR were 2.2 and 144, respectively. The cutoff values for the categorisation of the NLR into quintiles were 1.39, 2.00, 2.58, and 4.00. Patients in the lower NLR quintile (quintiles 1 and 2) were significantly older than those in the higher quintiles (Table 1). Chinese patients were more likely to have a high NLR compared with Indians. Tumour size was positively associated with the NLR. Higher NLR quintiles were more likely to be associated with lymph node involvement than the lower two quintiles. The proportion of patients with de novo metastatic breast cancer was remarkably higher in NLR quintile 5. Higher NLR quintiles were also significantly associated with unfavourable tumour characteristics including higher tumour grade, LVI, lack of PR expression, and HER2 expression. Importantly, the NLR and PLR were positively correlated; Pearson coefficient regression=0.586, P<0.001. In a multivariable logistic regression, only HER2 expression, increasing tumour size, and PLR were significantly associated with an elevated NLR. Patients in NLR quintile 5 were least likely to have undergone any surgery. In women subjected to surgery, those in NLR quintile 5 were most likely to have received neoadjuvant chemotherapy (Table 1).
The cutoff values for the categorisation of PLR into quintiles were 100, 129, 161, and 215. A higher PLR was significantly associated with a younger age at diagnosis (Table 2). Chinese patients were more likely to have a higher PLR than the Indians. Tumour size and lymph node involvement were positively associated with the PLR. Patients in PLR quintile 5 were significantly associated with higher stages, particularly stage IV disease. Their tumours were also more likely to be associated with poor prognostic features such as LVI and HER2 expression. Following multivariable logistic regression, Chinese ethnicity, high nodal burden (>9 nodes), distant metastasis, and an increasing NLR were associated with an elevated PLR. Patients in the higher PLR quintile were least likely to have undergone any surgery. A high neoadjuvant chemotherapy administration rate was also observed among patients in the higher PLR quintile who were candidates for surgery.
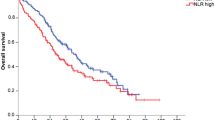
The RSR was highest in breast cancer patients in the lowest NLR quintile, whereas it was lowest in the highest NLR quintile (Figure 1). The 5-year RSRs were 76.4% (95% CI: 69.6–82.1%) in quintile 1, 79.4% (95% CI: 74.4–83.7%) in quintile 2, 72.1% (95% CI: 66.3–77.3%) in quintile 3, 65.6% (95% CI: 59.8–70.8%) in quintile 4, and 51.1% (95% CI: 43.3–58.5%) in quintile 5. The risk of death in patients in the highest NLR quintile was 2.5 times higher than for their counterparts in the lowest quintile (Table 3). In the multivariable analysis, both the NLR and PLR were found to be independently associated with survival. Patients in the highest NLR quintile remained significantly associated with a higher risk of mortality than those in the lowest quintile following adjustment for demography, tumour characteristics, treatment, and PLR; HR: 1.50 (95% CI: 10.8–1.63); P for the linear trend test=0.004.
Women with breast cancer in the highest quintile of the PLR were also found to have substantially lower survival rates than their counterparts in the other quintiles. The 5-year RSRs were 77.0% (95% CI: 70.9–82.2%) in quintile 1, 75.3% (95% CI: 69.4–80.5%) in quintile 2, 76.3% (95% CI: 70.4–81.4%) in quintile 3, 70.0% (95% CI: 64.0–75.4%) in quintile 4, and 53.2% (95% CI: 46.9–59.2%) in quintile 5 (Figure 2). Following multivariable adjustment for demographics, tumour characteristics, treatment, and the NLR, an increased PLR was no longer associated with an increased risk of death; the HR for quintile 5 was 1.07 (95% CI: 0.81–1.41) compared with quintile 1 (Table 3).
Sensitivity analyses using different cutoff levels for the NLR did not change the main results (Table 4). However, in a fully adjusted multivariable analysis, patients with a PLR >185 were significantly associated with a higher risk of death compared with their counterparts with a PLR ⩽185. Using a cutoff of 292, a high PLR was not significantly associated with a risk of mortality following breast cancer (Table 4).
The breast cancer subtype did not appear to modify the association between the NLR/PLR and survival; P-values for interaction were 0.147 and 0.680, respectively. Subgroup analysis by subtype of breast cancer showed that an elevated NLR was significantly associated with an increased mortality in women with ER− and PR− breast cancer, irrespective of HER2 status (Table 5). On the contrary, an elevated PLR was independently associated with an increased risk of mortality in patients with ER+ or PR+ and HER2+ breast cancer (Table 5).
Discussion
In this study, a higher pre-treatment NLR was significantly and independently associated with higher mortality in women with breast cancer, with evidence of a dose–response relationship. The results remained robust despite using different cutoff values. Elevation of the pre-treatment PLR was also independently associated with breast cancer mortality. However, there was no evidence of a dose–response relationship, and the results could not be replicated when different cutoff levels were used.
An elevated NLR is associated with adverse survival probabilities in gastrointestinal cancers; namely, colorectal, stomach, liver, oesophageal, and pancreatic cancers (Walsh et al, 2005; Shimada et al, 2010; Sharaiha et al, 2011; Chiang et al, 2012; Limaye et al, 2013; Stotz et al, 2013; Xu et al, 2014). A very recent meta-analysis of 40 559 patients with solid tumours found that an NLR greater than 4.00 was associated with a substantial increase in risk for all-cause mortality (HR: 1.81, 95% CI: 1.67–1.97) (Templeton et al, 2014b). Although evidence on the prognostic role of the NLR in breast cancer has been relatively scarce (Azab et al, 2012, 2013; Noh et al, 2013; Dirican et al, 2014; Nakano et al, 2014; Yao et al, 2014), our robust results add valuable evidence that the NLR is also an adverse prognostic indicator in breast cancer. An earlier study in breast cancer patients, which stratified the NLR according to quartiles, reported that mortality was higher in the highest NLR quartile but closely similar among the lower three quartiles, suggesting a threshold effect (Azab et al, 2012). In our study, although the risk of mortality increased substantially with each NLR quintile in univariable analysis, the observation that this trend was attenuated after multivariable adjustment seems to suggest that the association between the NLR and mortality in breast cancer may not be entirely linear.
It has been suggested that optimal cutoff values for prognostic markers may be better selected by validating previously established cutoff values from other cohort studies (Levine et al, 1991). We therefore performed sensitivity analyses using dichotomous categorisation of the NLR/PLR, adopting previously reported cutoff values (Krenn-Pilko et al, 2014; Templeton et al, 2014a, 2014b). On the basis of our findings, it seems that a cutoff value of 4.00 for the NLR (Dirican et al, 2014; Templeton et al, 2014b), which was also the cutoff value for patients in quintile 5 in this study, is able to distinguish between those with a higher risk of mortality and those with a lower risk. Nevertheless, the HRs for all-cause mortality in previous breast cancer-specific studies were higher than the observed HR in this study (Azab et al, 2012, 2013; Noh et al, 2013; Dirican et al, 2014; Nakano et al, 2014). A more recent study, however, did not find an association between NLR and disease-free survival, as well as overall survival (Cihan et al, 2014).
Although a previous study had shown that PLR was not associated with either disease-free survival or overall survival in women with breast cancer (Cihan et al, 2014), our finding that PLR was an adverse prognostic predictor in breast cancer (using a cutoff value of 185) corroborates the findings of Azab et al (2013). As in the analysis of the NLR, the HR of mortality in this previous study was substantially higher than in our current analysis (2.68, 95% CI: 1.61–4.46 vs 1.25, 95% CI: 1.04–1.52). Another recent study, which had used a cutoff value of 292, had also shown that the HR for all-cause mortality was higher than in our study (1.92, 95% CI: 1.01–3.67 vs 1.30, 95% CI: 0.98–1.70) (Krenn-Pilko et al, 2014). However, it is felt that a high cutoff point may miss a substantial number of patients in clinical practice, given that <10% of patients in the current study were grouped into a PLR >292.
The prognostic impact of the NLR and PLR (as reflected by the HR) that we have observed is lower than in previous breast cancer-specific studies, and may be explained by several factors. Given that both the NLR and PLR were mutually correlated, and independently associated with survival, it is important that they are adjusted against each other in the multivariable analysis. It was less clear whether this was done in other studies except in one (Azab et al, 2013). Furthermore, patients with an increased NLR/PLR were more likely to be associated with advanced disease stages, and unfavourable tumour characteristics. It is hence plausible that treatment patterns may also vary across the categories, as observed in our study. Only two of the six previous breast cancer-specific studies adjusted for adjuvant radiotherapy or chemotherapy (Azab et al, 2012; Dirican et al, 2014). Therefore, it remains possible that the higher HRs in the previous studies may be attenuated after adjustment for full treatment details.
Although previous studies had found that the impact of NLR/PLR on breast cancer prognosis varies according to breast cancer subtypes (Noh et al, 2013; Krenn-Pilko et al, 2014), we did not find significant effect modification. Furthermore, our results are not entirely in agreement with previous studies. We are hence uncertain whether our significant results were due to multiple testing.
The mechanism by which the NLR and PLR may impact breast cancer prognosis remains unclear. The peripheral NLR and PLR are thought to be proxies of the on-going inflammatory process in the tumour microenvironment. A complex body of scientific evidence suggests that neutrophils and platelets are associated with pro-tumour activities in vivo such as enhanced angiogenesis, which contribute to tumour cell proliferation and promote metastatic potential of the tumour cells (Coussens and Werb, 2002; De Larco et al, 2004; Bambace and Holmes, 2011; Voutsadakis, 2014). Lymphocytes, on the other hand, have been implicated in having an important role in cancer immune surveillance, and are hypothesised to suppress tumour maturation (Shankaran et al, 2001). An increased concentration of intratumoral CD8+ cytotoxic lymphocytes in breast cancer has been strongly associated with decreased recurrence, and higher survival outcomes (Mahmoud et al, 2011). It is hence biologically plausible that imbalances in the ratio of the peripheral neutrophils/platelets to lymphocytes may provide an insight into underlying tumour progression and prognosis in individuals with breast cancer. This seems to further suggest that the NLR and PLR may also have the potential to be predictive markers in breast cancer.
To our knowledge, this is the largest study to have investigated the prognostic role of the pre-treatment NLR/PLR in an unselected cohort of women with breast cancer, of which a high proportion of patients (∼70%) had available information on the NLR/PLR. A major strength of this study is that we had detailed information on tumour characteristics and treatment, allowing for extensive confounder adjustment. Although we did not have information on the cause of death in our Registry, we had estimated RSRs, which provide an estimate of net survival attributed to breast cancer, given that it captures both the direct and indirect contribution of cancer diagnosis on survival (Coleman et al, 2008). However, (relative) survival may have been slightly overestimated in the current study, as breast cancer is more common in affluent women (Clarke et al, 2002), making life expectancies between the patients and the background population not entirely comparable. Given that we had compared all-cause mortality in the multivariable analysis, it is acknowledged that lack of data on patients’ comorbidities may have affected our study results.
Conclusion
In conclusion, our results support the findings of previous studies that an increased NLR and PLR are independently associated with a higher risk of all-cause mortality in women with breast cancer. This association does not seem to be modified by the subtype of breast cancer. Given that the NLR and PLR are readily available biomarkers in clinical settings, future prognostic studies are warranted to determine the added value of these biomarkers to existing prognostic indicators of breast cancer that are routinely used in clinical practice, and also on their potential as predictive markers in breast cancer.
References
Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD (2012) Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 19: 217–224.
Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, Habeshy A, Picon A, Bloom S (2013) Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol 30: 432.
Bambace NM, Holmes CE (2011) The platelet contribution to cancer progression. J Thromb Haemost 9: 237–249.
Chiang S-F, Hung H-Y, Tang R, Changchien CR, Chen J-S, You Y-T, Chiang J-M, Lin J-R (2012) Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis 27: 1347–1357.
Cihan YB, Arslan A, Cetindag MF, Mutlu H (2014) Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer. Asian Pac J Cancer Prev 15: 4225–4231.
Clarke C a, Glaser SL, West DW, Ereman RR, Erdmann CA, Barlow JM, Wrensch MR (2002) Breast cancer incidence and mortality trends in an affluent population: Marin County, California, USA, 1990-1999. Breast Cancer Res. 4: R13.
Coleman MP, Quaresma M, Berrino F, Lutz J-M, De Angelis R, Capocaccia R, Baili P, Rachet B, Gatta G, Hakulinen T, Micheli A, Sant M, Weir HK, Elwood JM, Tsukuma H, Koifman SE, Silva GA, Francisci S, Santaquilani M, Verdecchia A, Storm HH, Young JL (2008) Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 9: 730–756.
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860–867.
DeNardo DG, Coussens LM (2007) Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res 9: 212.
Dickman PW, Sloggett A, Hills M, Hakulinen T (2004) Regression models for relative survival. Stat Med 23: 51–64.
Dirican A, Kucukzeybek BB, Alacacioglu A, Kucukzeybek Y, Erten C, Varol U, Somali I, Demir L, Bayoglu IV, Yildiz Y, Akyol M, Koyuncu B, Coban E, Ulger E, Unay FC, Tarhan MO (2014) Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol 20: 70–81.
Gregory AD, Houghton A M (2011) Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res 71: 2411–2416.
Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ (2013) The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 88: 218–230.
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674.
Krenn-Pilko S, Langsenlehner U, Thurner E-M, Stojakovic T, Pichler M, Gerger A, Kapp KS, Langsenlehner T (2014) The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer 110: 2524–2530.
Lal I, Dittus K, Holmes CE (2013) Platelets, coagulation and fibrinolysis in breast cancer progression. Breast Cancer Res 15: 207–218.
De Larco JE, Wuertz BRK, Furcht LT (2004) The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 10: 4895–4900.
Levine MN, Browman GP, Gent M, Roberts R, Goodyear M (1991) When is a prognostic factor useful?: A guide for the perplexed. J Clin Oncol 9: 348–356.
Li M-X, Liu X-M, Zhang X-F, Zhang J-F, Wang W-L, Zhu Y, Dong J, Cheng J-W, Liu Z-W, Ma L, Lv Y (2014) Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 134: 2403–2413.
Limaye AR, Clark V, Soldevila-Pico C, Morelli G, Suman A, Firpi R, Nelson DR, Cabrera R (2013) Neutrophil-lymphocyte ratio predicts overall and recurrence-free survival after liver transplantation for hepatocellular carcinoma. Hepatol Res 43: 757–764.
Mahmoud SM a, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AHS, Ellis IO, Green AR (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29: 1949–1955.
Nakano K, Hosoda M, Yamamoto M, Yamashita H (2014) Prognostic significance of pre-treatment neutrophil: lymphocyte ratio in japanese patients with breast cancer. Anticancer Res 34: 3819–3824.
Noh H, Eomm M, Han A (2013) Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer 16: 55–59.
Pathy NB, Yip CH, Taib NA, Hartman M, Saxena N, Iau P, Bulgiba AM, Lee SC, Lim SE, Wong JEL, Verkooijen HM (2011) Breast cancer in a multi-ethnic Asian setting: results from the Singapore-Malaysia hospital-based breast cancer registry. Breast 20 (Suppl 2): S75–S80.
Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DSJ, Foulis AK, Horgan PG, McMillan DC (2011) A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 47: 2633–2641.
Roxburgh CSD, McMillan DC (2010) Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 6: 149–163.
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD (2001) IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410: 1107–1111.
Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA (2011) Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 18: 3362–3369.
Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M (2010) High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 13: 170–176.
Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M (2013) Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 109: 416–421.
Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, Seruga B, Ocaña A, Tannock IF, Amir E (2014a) Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 23: 1204–1212.
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E (2014b) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst 106: 1–11.
Voutsadakis IA (2014) Thrombocytosis as a prognostic marker in gastrointestinal cancers. World J Gastrointest Oncol 6: 34–40.
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91: 181–184.
Xu A, Huang L, Zhu L, Wei Z (2014) Significance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 cases. Am J Cancer Res 4: 189–195.
Yao M, Liu Y, Jin H, Liu X, Lv K, Wei H, Du C, Wang S, Wei B, Fu P (2014) Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther 7: 1743–1752.
Acknowledgements
This study was financially supported by the Ministry of Education Malaysia (High Impact Research Grant (UM.C/HIR/MOHE/06)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Disclaimer
The funder did not have any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Rights and permissions
This work is licensed under the Creative Commons Attribution-Non-Commercial-Share Alike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Koh, CH., Bhoo-Pathy, N., Ng, KL. et al. Utility of pre-treatment neutrophil–lymphocyte ratio and platelet–lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer 113, 150–158 (2015). https://doi.org/10.1038/bjc.2015.183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.183
Keywords
This article is cited by
-
The prevalence, hospitalization outcomes and risk factors of euthyroid sick syndrome in patients with diabetic ketosis/ketoacidosis
BMC Endocrine Disorders (2023)
-
Diagnostic efficacy of systemic immune-inflammation biomarkers in benign prostatic hyperplasia using receiver operating characteristic and artificial neural network
Scientific Reports (2023)
-
Prognostic analysis of stereotactic radiosurgery for brain metastases: a single-center retrospective study
La radiologia medica (2023)
-
Inflammation-scores as prognostic markers of overall survival in lung cancer: a register-based study of 6,210 Danish lung cancer patients
BMC Cancer (2022)
-
High platelet-to-lymphocyte ratios in triple-negative breast cancer associates with immunosuppressive status of TILs
Breast Cancer Research (2022)