Abstract
Background:
Pertuzumab plus trastuzumab provides a more comprehensive blockade of HER2 signalling than trastuzumab alone. Therefore, we conducted a phase IIa study of the pharmacokinetics and safety of pertuzumab plus trastuzumab and chemotherapy in advanced gastric cancer (aGC).
Methods:
Patients received pertuzumab 840 mg for cycle 1 and 420 mg q3w for cycles 2–6 (Arm A) or pertuzumab 840 mg q3w for six cycles (Arm B). Trastuzumab, cisplatin and capecitabine were also given for six cycles, then trastuzumab q3w until disease progression or unmanageable toxicity. The co-primary endpoints were day 43 pertuzumab serum trough concentration (Cmin) and safety.
Results:
Thirty patients were randomised. Mean pertuzumab Cmin at day 43 was 40.0 μg ml−1 (s.d.: 17.3) in Arm A and 62.7 μg ml−1 (29.1) in Arm B. Mean day 43 Cmin in Arm A was ∼37% lower than that seen in metastatic breast cancer. The safety profiles were similar between arms and treatment was well tolerated. Partial responses were achieved by 86% and 55% of patients in Arms A and B, respectively.
Conclusions:
On the basis of the pharmacokinetic and safety data, the 840 mg q3w pertuzumab dose has been selected for a phase III study of pertuzumab, trastuzumab and chemotherapy in HER2-positive aGC.
Similar content being viewed by others
Main
Globally, gastric cancer is the fourth most commonly occurring cancer (Ferlay et al, 2010), with variable overall survival rates; the 5-year survival rate ranges from 10–30% in most of the world to 50–70% in Korea and Japan (Howlader et al, 2011; Matsuda and Saika, 2013; Jung et al, 2013a; De Angelis et al, 2014). Although surgical resection of localised gastric tumours offers a potentially curative therapy, most patients present with inoperable, advanced or metastatic disease requiring palliative treatment (Dicken et al, 2005), with the exception of some countries in East Asia, such as Korea and Japan, where national screening programmes are conducted (Dicken et al, 2005; Jung et al, 2013b). Combining multiple chemotherapy agents has improved overall survival rates compared with single agents or best supportive care (Wagner et al, 2006), and the current standard chemotherapy regimen for patients with advanced gastric cancer (aGC) contains a fluoropyrimidine plus a platinum compound (Jackson et al, 2009). However, the prognosis of patients with aGC remains poor and improved treatment options are required for this disease.
Human epidermal growth factor receptor 2 (HER2) overexpression/amplification is an established negative prognostic biomarker in breast cancer and occurs in ∼20% of cases (Slamon et al, 1987, 1989), whereas 17–22% of gastric cancer cases have been reported to be HER2-positive (Tanner et al, 2005; Lordick et al, 2007; Zhang et al, 2009; Bang et al, 2010). In the phase III ToGA trial, the addition of the HER2-targeted humanised monoclonal antibody trastuzumab to first-line fluoropyrimidine plus cisplatin chemotherapy significantly increased overall survival in patients with HER2-positive advanced gastric or gastro–oesophageal junction cancer, with further survival benefit in patients whose tumours had high levels of HER2 expression (immunohistochemistry (IHC) 3+ or IHC 2+ and fluorescence in situ hybridisation-positive; Bang et al, 2010). The grade 3–4 adverse event (AE) profiles were similar between the trastuzumab plus chemotherapy arm and the chemotherapy-only arm, with the exception of increased rates of diarrhoea in patients receiving trastuzumab (Bang et al, 2010). On the basis of the data from the ToGA trial, trastuzumab was approved in combination with capecitabine plus cisplatin or 5-fluorouracil plus cisplatin for the first-line treatment of patients with HER2-positive metastatic adenocarcinoma of the stomach or gastro–oesophageal junction (Herceptin Summary of Product Characteristics, 2013). Lapatinib, an oral tyrosine kinase inhibitor of epidermal growth factor receptor and HER2, has also been tested in HER2-positive gastric cancer. However, lapatinib did not improve overall survival when combined with chemotherapy for first-line (Hecht et al, 2013) or second-line treatment (Bang et al, 2013) of HER2-positive aGC.
Pertuzumab is a humanised monoclonal antibody that targets a different epitope of HER2 from trastuzumab (Cho et al, 2003; Franklin et al, 2004). Whereas trastuzumab binds to subdomain IV of HER2 (Cho et al, 2003) to inhibit ligand-independent signalling (Junttila et al, 2009), pertuzumab binds to subdomain II (Franklin et al, 2004), the dimerisation domain, to inhibit HER2 heterodimerisation and, consequently, ligand-dependent signalling (Agus et al, 2002). Pre-clinical studies have suggested that trastuzumab and pertuzumab have complementary mechanisms of action and that the combination of the two antibodies provides a more efficient blockade of HER2 signalling than either antibody alone (Nahta et al, 2004; Scheuer et al, 2009). In the CLEOPATRA study of patients with HER2-positive metastatic breast cancer (MBC), the combination of pertuzumab, trastuzumab and docetaxel significantly improved progression-free (Baselga et al, 2012) and overall survival (Swain et al, 2013) compared with placebo, trastuzumab and docetaxel. Furthermore, pre-clinical studies of a HER2-positive human gastric cancer xenograft model showed enhanced anti-tumour activity when pertuzumab and trastuzumab were combined, compared with that seen with either antibody alone (Yamashita-Kashima et al, 2011). Therefore, the combination of pertuzumab with trastuzumab and chemotherapy has the potential to improve survival outcomes in patients with HER2-positive aGC compared with trastuzumab and chemotherapy alone.
In patients with HER2-positive aGC, the observed steady-state exposure of trastuzumab was lower than that seen with an identical dose in MBC, and patients with trastuzumab concentrations in the lowest quartile had a shorter median overall survival (7.7 (range, 6.3–10.6) months) than patients in the upper three quartiles (15.7 (range, 14.1–18.9) months; Yang et al, 2013). Furthermore, in a separate analysis, patients with the lowest trastuzumab serum trough concentrations had the highest rate of disease progression and shortest overall survival (Cosson et al, 2014). Therefore, we aimed to study the pharmacokinetics (PK) of pertuzumab in combination with trastuzumab and chemotherapy to identify the pertuzumab dose that produces a steady-state trough serum concentration (Cmin) of ⩾20 μg ml−1 in at least 90% of patients with HER2-positive gastric cancer. This PK target was derived from dose-response xenograft studies of pertuzumab administered as a single agent showing effective tumour-growth inhibition with serum trough concentrations of 5–25 μg ml−1 (Malik et al, 2003), and is also the PK target that was used to determine the pertuzumab dose in breast cancer. In patients with HER2-positive breast cancer, the PK target was achieved with a pertuzumab loading dose of 840 mg followed by 420 mg q3w, a dosing regimen that has shown promising activity in early and advanced breast cancer treatment settings (Baselga et al, 2010; Cortés et al, 2012; Gianni et al, 2012; Schneeweiss et al, 2013; Swain et al, 2013). Importantly, phase I/II trials of both weight-based and fixed pertuzumab doses in multiple tumour types have not revealed a maximum tolerated dose (Agus et al, 2005; Attard et al, 2007; Albanell et al, 2008). Here we present the results of JOSHUA, a phase IIa study of the PK and safety of two different doses of pertuzumab in combination with trastuzumab and chemotherapy, to identify the optimal pertuzumab dose for clinical studies in HER2-positive gastric cancer.
Materials and methods
Study design and treatment
JOSHUA was a randomised, multicentre, open-label phase IIa trial (NCT01461057) evaluating two different doses of pertuzumab in patients with HER2-positive advanced gastric or gastro–oesophageal junction cancer. Treatment was given in 3-weekly cycles. Patients were randomised to receive an initial pertuzumab dose of 840 mg for cycle 1, followed by a dose of 420 mg for cycles 2–6 (Arm A) or pertuzumab 840 mg for cycles 1–6 (Arm B). Patients in both treatment arms received cisplatin (80 mg m−2) and capecitabine (1000 mg m−2 BID for 14 days) for cycles 1–6; in the absence of disease progression or unmanageable toxicity, patients could receive capecitabine for more than six cycles, at the discretion of the investigator. All patients received trastuzumab at 8 mg kg−1 for cycle 1, followed by 6 mg kg−1 for subsequent cycles, until investigator-assessed disease progression or unmanageable toxicity. The study was approved by the institutional review board of each participating centre or the competent authority and ethics committee. The study was conducted in full accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent.
The co-primary endpoints of the study were Cmin at day 43 (cycle 2 trough concentration) and safety. The aim of the PK endpoint was to identify the pertuzumab dose that produced a steady-state Cmin of ⩾20 μg ml−1 in at least 90% of patients. The aim of the safety endpoint was to assess the incidence of all AEs. An exploratory objective of the study was to assess the anti-tumour activity of pertuzumab in combination with trastuzumab and chemotherapy in this disease setting.
Patient population
Patients ⩾18 years old were eligible if they had histologically confirmed, inoperable, locally advanced or metastatic HER2-positive adenocarcinoma of the stomach or gastro–oesophageal junction. HER2 positivity was defined as IHC 3+ or IHC 2+ and in situ hybridisation (ISH)-positive (ISH positivity was defined as a HER2 : CEP17 signal ratio of ⩾2.0) by central testing. Eligible patients had measurable disease or non-measurable, evaluable disease according to Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 (Eisenhauer et al, 2009). Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 or 1, a baseline left ventricular ejection fraction (LVEF) of ⩾55% and a life expectancy of ⩾3 months. Patients were eligible if they had not received prior treatment for advanced or metastatic disease; prior (neo)adjuvant therapy was allowed if completed ⩾6 months before study enrolment, whereas platinum-based (neo)adjuvant therapy was prohibited. Patients were not eligible for the study if they had a serious cardiac illness or medical condition.
Analysis of PK
For pertuzumab PK assessment, pre-dose and post-dose serum samples were collected at cycles 1, 2, 3, 4 and 6. Pre-dose samples were collected up to 6 h before the administration of pertuzumab and post-dose samples were collected up to 30 min after the pertuzumab infusion. The cycle 2 trough (cycle 3 pre-dose) sample had to be collected on study day 43 (21 days after the cycle 2 dose), regardless of whether the cycle 3 dose was given, delayed or not given. In addition, weekly serum samples were collected in cycles 1 and 2. A validated bridging enzyme-linked immunosorption assay was used to measure the concentration of pertuzumab in serum samples. The assay used a monoclonal anti-idiotype antibody to capture pertuzumab and had a minimum quantifiable concentration in human serum of 150 ng ml−1.
Assessments
For tumour response assessments, all measurable and non-measurable, evaluable disease had to be documented at screening and reassessed at the end of cycles 3 and 6 according to RECIST v1.1, after which patients were followed for tumour response according to local standards. Response was assessed by the investigator based on physical examinations, computed tomography scans or magnetic resonance imaging. Other methods were allowed if they were amenable to evaluation per RECIST.
LVEF assessments by echocardiogram or multiple-gated acquisition scan were performed at baseline and then every three cycles until disease progression or treatment discontinuation. LVEF assessments were performed at the treatment discontinuation visit, every 6 months for the first year following treatment discontinuation, then annually for up to 3 years following treatment discontinuation. Patients who discontinued study treatment permanently due to LVEF decline continued to have LVEF assessments repeated as clinically indicated, with a maximum interval of 3 months between assessments, until LVEF returned to >50%, or 1 year after the treatment discontinuation visit, whichever occurred first. Thereafter, LVEF assessments were performed annually for up to 3 years after the treatment discontinuation visit.
AEs were graded according to The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0 (National Cancer Institute, Bethesda, MD, USA). Real-time safety monitoring occurred on an ongoing basis for all patients and expedited reporting was performed for serious AEs and AEs of special interest. AEs and serious AEs were recorded throughout the study and up to 6 months after the last dose of study treatment, with the exception of cardiac AEs, which were followed for 12 months after the last dose of study treatment, and symptomatic LVEF, which was required to be reported up to 3 years after the last dose of study treatment. Study drug-related serious AEs continued to be recorded regardless of the time elapsed since the last dose of study treatment.
Statistical analysis
A sample size of 15 patients per arm was considered sufficient to determine the dose needed to achieve the target Cmin with an acceptable degree of precision (coefficient of variation <15%). This was determined based on trastuzumab PK data from the ToGA trial and the assumption that pertuzumab behaves similarly to trastuzumab in aGC. However, due to variability, outliers and the limited number of data points due to patient withdrawals, it was not possible to accurately assess the primary PK endpoint on the basis of the observed percentage of patients who achieved the target Cmin. Therefore, a post hoc bootstrap analysis was conducted to estimate the percentage of patients at or above the target Cmin and the confidence interval (CI) of this estimate (analysis performed in R Statistics Software version 2.10.1, R Foundation for Statistical Computing, Vienna, Austria). For each pertuzumab dose group, a sample of 15 patients was generated on the basis of the mean and s.d. of the observed data assuming a log-normal distribution, and the percentage of patients with a pertuzumab Cmin of ⩾20 μg ml−1 was computed. The process was repeated 1000 times to generate an estimated percentage of patients with a pertuzumab Cmin of ⩾20 μg ml−1 and a 95% CI.
Results
Patient and disease characteristics
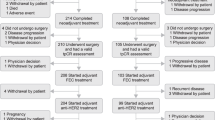
Between December 2011 and April 2012, 30 patients enroled into the study and 15 were randomised into each arm. The baseline demographics and disease characteristics of patients were generally well balanced between arms, although there were some imbalances in terms of age and sex, with older patients and more men in Arm A (Table 1). The majority of patients had metastatic gastric cancer.
The data cutoff occurred on 31 December 2012, at which point the mean follow-up time was 312 days and 260 days in Arms A and B, respectively. Fourteen patients in Arm A and 11 patients in Arm B had received all six cycles of pertuzumab (Table 2). Seven patients in Arm A and three patients in Arm B were still receiving study treatment while the remaining patients had discontinued: eight patients in Arm A (due to disease progression) and 12 patients in Arm B (eight due to disease progression, one due to an AE (worsening renal failure), one at the physician’s decision (due to pneumonia), one death (fungal pneumonia) and one withdrawal by the patient).
PK of pertuzumab
Mean pertuzumab serum concentration–time profiles are shown in Figure 1. Pertuzumab concentrations were similar between arms at cycle 1 (when the dose was the same for each arm). However, from cycle 2 onwards, pertuzumab concentrations were higher in Arm B than in Arm A, consistent with the higher dose in Arm B. In Arm A, the mean pertuzumab Cmin increased ∼2-fold from day 22 to day 106, whereas in Arm B the increase in mean pertuzumab Cmin over this time was slightly higher, at ∼2.9-fold. Overall, pertuzumab concentrations were dose proportional.
Fifteen patients in Arm A and 13 patients in Arm B had data available to assess pertuzumab Cmin at day 43. One patient in Arm B had abnormally low pertuzumab concentrations, with a day 22 Cmin below the assay’s lower limit of quantification and a day 43 Cmin of 0.610 μg ml−1. After excluding this patient from the analysis, the day 43 mean (s.d.) pertuzumab Cmin in Arms A and B was 40.0 μg ml−1 (17.3) and 62.7 μg ml−1 (29.1), respectively, and the geometric mean Cmin in Arms A and B was 36.8 μg ml−1 and 56.4 μg ml−1, respectively.
Summary box-scatter plots in Figure 2 show the day 43 pertuzumab Cmin values observed in this study and in the pertuzumab arm of the CLEOPATRA trial, which studied HER2-positive MBC. Mean pertuzumab Cmin with the 840/420 mg dose in HER2-positive aGC (Arm A) was ∼37% lower than that observed with the same dose in HER2-positive MBC (in CLEOPATRA). Mean pertuzumab Cmin with the 840 mg q3w dose in HER2-positive aGC (Arm B) was similar to that observed with the 840/420 mg dose in HER2-positive MBC.
Comparison of pertuzumab trough concentrations in JOSHUA (HER2-positive aGC) with CLEOPATRA (HER2-positive MBC). Summary box-scatter plots of day 43 pertuzumab trough concentrations observed in JOSHUA and CLEOPATRA (pertuzumab arm). Black solid line=median; box limits=25th–75th percentiles; whiskers=1.5 × interquartile range; circles=individual concentrations; dotted line=20 μg ml−1 target.
As day 43 pertuzumab Cmin samples from Arm B were only available for 12 patients, the ability to select the most appropriate pertuzumab dose based on the observed data was limited. Therefore, a bootstrap analysis was conducted to estimate the percentage of patients who achieved the target pertuzumab Cmin of ⩾20 μg ml−1 at day 43 and the CI of this estimate. This analysis showed that 91.6% (95% CI 78.3, 99.2) and 98.3% (95% CI 91.4, 99.97) of patients in Arms A and B, respectively, achieved the target Cmin.
Safety
At the time of data cutoff, patients in Arm A had received a median of six (range, 4–6) doses of pertuzumab and patients in Arm B had received a median of six (range, 1–6) doses of pertuzumab. Overall, the AE profiles were similar between arms. Diarrhoea was the most common AE and was mostly grade 1–2; onset was in cycle 1, with the frequency decreasing during subsequent cycles. Figure 3 shows the incidence of diarrhoea at each treatment cycle. No patient discontinued therapy due to diarrhoea. There were 24 reports (16 in Arm A and 8 in Arm B) of grade ⩾2 diarrhoea in 18 patients (11 in Arm A and 7 in Arm B). Onset of the first incidence of grade ⩾2 diarrhoea in these patients occurred on median (range) study day 12 (1–237) (study day 13 (6–237) in Arm A, study day 8 (1–16) in Arm B). There were 13 instances (8 in Arm A (in seven patients) and 5 in Arm B (in three patients)) of capecitabine dose reduction due to an AE that occurred either during the duration of grade ⩾2 diarrhoea or on day 1 of the subsequent treatment cycle, following resolution of grade ⩾2 diarrhoea. For these 10 patients, initial capecitabine dose reduction occurred on median (range) study day 35.5 (22–181) (study day 42 (23–181) in Arm A, study day 22 (22–56) in Arm B).
The total number of grade ⩾3 AEs was 78 in Arm A and 60 in Arm B and the most frequent grade ⩾3 AEs are shown in Table 3. Seventy-three percent and 67% of patients in Arms A and B, respectively, experienced at least one serious AE and the total number of all serious AEs was 35 in Arm A and 19 in Arm B. Serious AEs that occurred in ⩾2 patients overall were diarrhoea, febrile neutropenia, acute renal failure, asthenia, fatigue, gastric obstruction, hyponatraemia, mucosal inflammation, neutropenia, pneumonia, pulmonary embolism and vomiting. One serious AE in Arm B (fungal pneumonia) resulted in death. This was considered by the investigator to be related to the chemotherapy agents. No patient experienced symptomatic heart failure. Two patients, both in Arm A, experienced asymptomatic LVEF decline. After delaying study treatment, their LVEF values recovered and these patients continued receiving study treatment.
Overall response
An exploratory endpoint of the study was to assess the anti-tumour activity of pertuzumab with trastuzumab, cisplatin and capecitabine in patients with HER2-positive aGC (Table 4). At the end of cycle 3, 10 (67%) and 7 (58%) patients in Arms A and B, respectively, had achieved a partial response, whereas an additional 4 patients in each arm (27% and 33%, respectively) had stable disease. One patient in each arm had progressive disease. At the end of cycle 6, a partial response was observed in 12 (86%) and 6 (55%) patients in Arms A and B, respectively, whereas 2 (14%) and 3 (27%) patients, respectively, had stable disease. Two patients in Arm B had progressive disease.
Discussion
The primary objective of this study was to identify the pertuzumab dose that produces a steady-state concentration of ⩾20 μg ml−1 in at least 90% of patients with HER2-positive aGC. The PK data show that, as expected, the 840 mg q3w dose resulted in higher pertuzumab concentrations overall than the 840/420 mg dose. The data demonstrate that both doses resulted in day 43 trough concentrations above the target of 20 μg ml−1 in at least 90% of patients; however, the lower bound of the 95% CI in Arm A in the bootstrap analysis was 78.3%, suggesting a greater risk of not achieving the PK target in at least 90% of patients with the 840/420 mg pertuzumab dose and that the 840 mg q3w pertuzumab dose is more likely to maintain trough concentrations above the target in at least 90% of patients. Moreover, the 840 mg q3w pertuzumab dose produced trough concentrations in patients with HER2-positive aGC similar to those observed in HER2-positive MBC in CLEOPATRA, whereas the 840/420 mg dose produced lower trough concentrations. Exposure-response analysis of data from the ToGA trial in aGC showed that patients with the lowest trastuzumab serum trough concentrations had the highest rate of disease progression and shortest overall survival (Yang et al, 2013; Cosson et al, 2014). Therefore, the 840 mg q3w pertuzumab dose is expected to provide greater treatment benefit than the 840/420 mg dose in patients with HER2-positive aGC.
Many population PK models have found patient demographic factors, such as age, body weight and gender, and also receptor number and disease-related factors, such as number of metastatic sites, circulating tumour receptors and tumour burden, to have a statistically significant impact on PK (Bruno et al, 2005; Gupta et al, 2012; Zhu et al, 2014). However, exploratory analysis did not show any apparent correlation between PK and these covariates in the small sample size of our study. Furthermore, no PK differences were observed between Asian and non-Asian patients, consistent with previous analyses (Dirks and Meibohm, 2010; Chiba et al, 2014; data not shown). As such, the reasons for the lower pertuzumab concentrations in aGC compared with MBC are currently unknown.
At this interim safety assessment, the combination of pertuzumab, trastuzumab and chemotherapy was well tolerated. The AE profiles were similar between arms and consistent with those observed with trastuzumab and chemotherapy in the ToGA trial (Bang et al, 2010) with the exception of higher rates of diarrhoea.
Events of diarrhoea in JOSHUA were mostly grade 1–2 and occurred early during treatment, with their frequency decreasing during subsequent cycles, particularly following cycle 6. Diarrhoea was manageable and no patient discontinued study treatment due to this AE. Although more patients discontinued treatment in Arm B than Arm A (12 vs 8 patients), it is not clear whether this was due to the higher pertuzumab dose, as AEs leading to discontinuation were not uniform. As a pertuzumab dose of 1050 mg q3w has been shown to be safe and tolerable in phase I/II studies in a variety of solid tumour types (Gordon et al, 2006; Attard et al, 2007; de Bono et al, 2007; Albanell et al, 2008; Gianni et al, 2010), the similarity of the safety profiles of the two doses in JOSHUA is not unexpected.
Preliminary data from the exploratory efficacy analysis show that patients with HER2-positive aGC treated with the combination of pertuzumab, trastuzumab, capecitabine and cisplatin achieved high rates of partial response (86% in Arm A and 55% in Arm B) or stable disease (14% in Arm A and 27% in Arm B) at the end of cycle 6. The overall response rates observed in JOSHUA compare favourably with that seen in patients with HER2-positive aGC treated with trastuzumab, capecitabine/fluorouracil and cisplatin in the ToGA trial (47%; Bang et al, 2010). Due to the small sample size and lack of a comparator arm in JOSHUA, no firm conclusions can be drawn about the activity of the study drug regimen or the difference between response rates in the two dose groups. However, results from JOSHUA support further investigation of dual blockade anti-HER2 therapy with pertuzumab plus trastuzumab in HER2-positive aGC.
In summary, a pertuzumab dose of 840 mg q3w in patients with HER2-positive aGC produces trough concentrations comparable to those seen in HER2-positive MBC without increasing the incidence of AEs. Therefore, the 840 mg q3w pertuzumab dose has been selected for an ongoing phase III study of first-line pertuzumab, trastuzumab and chemotherapy in HER2-positive metastatic gastric and gastro–oesophageal junction cancer (JACOB, NCT01774786; Hoff et al, 2013).
Change history
12 August 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, Press MF, Allison DE, Sliwkowski MX, Lieberman G (2005) Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol 23: 2534–2543.
Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX (2002) Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2: 127–137.
Albanell J, Montagut C, Jones ET, Pronk L, Mellado B, Beech J, Gascon P, Zugmaier G, Brewster M, Saunders MP, Valle JW (2008) A phase I study of the safety and pharmacokinetics of the combination of pertuzumab (rhuMab 2C4) and capecitabine in patients with advanced solid tumors. Clin Cancer Res 14: 2726–2731.
Attard G, Kitzen J, Blagden SP, Fong PC, Pronk LC, Zhi J, Zugmaier G, Verweij J, de Bono JS, de Jonge M (2007) A phase Ib study of pertuzumab, a recombinant humanised antibody to HER2, and docetaxel in patients with advanced solid tumours. Br J Cancer 97: 1338–1343.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697.
Bang Y-J, Xu R, Satoh T, Yeh K-H, Katsura K, Yoshida P, Mukaiyama A, Kobayashi M, Ohtsu A (2013) A randomized, open-label, phase III study of lapatinib in combination with weekly paclitaxel versus weekly paclitaxel alone in the second-line treatment of HER2 amplified advanced gastric cancer (AGC) in Asian population: Tytan study. J Clin Oncol 31 (February 1 suppl): Abstr 11.
Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM CLEOPATRA Study Group (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366: 109–119.
Baselga J, Gelmon KA, Verma S, Wardley A, Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA, Fumoleau P, Gianni L (2010) Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 28: 1138–1144.
Bruno R, Washington CB, Lu J, Lieberman G, Banken L, Klein P (2005) Population pharmacokinetics of trastuzumab in patients with HER2 metastatic breast cancer. Cancer Chemother Pharmacol 56: 361–369.
Chiba K, Yoshitsugu H, Kyosaka Y, Iida S, Yoneyama K, Tanigawa T, Fukushima T, Hiraoka M (2014) A comprehensive review of the pharmacokinetics of approved therapeutic monoclonal antibodies in Japan: are Japanese phase I studies still needed? J Clin Pharmacol 54: 483–494.
Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Leahy DJ (2003) Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421: 756–760.
Cortés J, Fumoleau P, Bianchi GV, Petrella TM, Gelmon K, Pivot X, Verma S, Albanell J, Conte P, Lluch A, Salvagni S, Servent V, Gianni L, Scaltriti M, Ross GA, Dixon J, Szado T, Baselga J (2012) Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 30: 1594–1600.
Cosson VF, Ng VW, Lehle M, Lum BL (2014) Population pharmacokinetics and exposure-response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Cancer Chemother Pharmacol 73: 737–747.
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E, Bielska-Lasota M, Engholm G, Nennecke A, Siesling S, Berrino F, Capocaccia R (2014) Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol 15: 23–34.
de Bono JS, Bellmunt J, Attard G, Droz JP, Miller K, Flechon A, Sternberg C, Parker C, Zugmaier G, Hersberger-Gimenez V, Cockey L, Mason M, Graham J (2007) Open-label phase II study evaluating the efficacy and safety of two doses of pertuzumab in castrate chemotherapy-naive patients with hormone-refractory prostate cancer. J Clin Oncol 25: 257–262.
Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM (2005) Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 241: 27.
Dirks NL, Meibohm B (2010) Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49: 633–659.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228–247.
Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893–2917.
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5: 317–328.
Gianni L, Llado A, Bianchi G, Cortes J, Kellokumpu-Lehtinen PL, Cameron DA, Miles D, Salvagni S, Wardley A, Goeminne JC, Hersberger V, Baselga J (2010) Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28: 1131–1137.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13: 25–32.
Gordon MS, Matei D, Aghajanian C, Matulonis UA, Brewer M, Fleming GF, Hainsworth JD, Garcia AA, Pegram MD, Schilder RJ, Cohn DE, Roman L, Derynck MK, Ng K, Lyons B, Allison DE, Eberhard DA, Pham TQ, Dere RC, Karlan BY (2006) Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: potential predictive relationship with tumor HER2 activation status. J Clin Oncol 24: 4324–4332.
Gupta M, LoRusso PM, Wang B, Yi J, Burris HA 3rd, Beeram M, Modi S, Chu Y, Agresta S, Klencke B (2012) Clinical implications of pathophysiological and demographic covariates on the population pharmacokinetics of trastuzumab emtansine, a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer. J Clin Pharmacol 52: 691–703.
Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK (eds) (2011) SEER Cancer Statistics Review, 1975-2008. National Cancer Institute: Bethesda, MD, USA http://seer.cancer.gov/csr/1975_2008/ (based on November 2010 SEER data submission, posted to the SEER web site.
Hecht JR, Bang Y, Qin S, Chung H, Xu J, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero AF, Salman P, Li J, Protsenko S, Buyse ME, Afenjar K, Kaneko T, Kemner A, Santillana S, Press MF, Slamon DJ (2013) Lapatinib in combination with capecitabine plus oxaliplatin (CapeOx) in HER2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma (AC): The TRIO-013/LOGiC Trial. J Clin Oncol 31 (June 20 suppl): LBA4001.
Herceptin Summary of Product Characteristics (2013) Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000278/WC500074922.pdf.
Hoff P, Tabernero J, Shen L, Ohtsu A, Yu R, Szado T, Kang Y (2013) Pertuzumab, trastuzumab and chemotherapy in HER2-positive metastatic gastric or gastro-oesophageal junction cancer: an international phase III study (JACOB). Ann Oncol 24 (suppl 4): iv67.
Jackson C, Cunningham D, Oliveira J (2009) Gastric cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20 (suppl 4): 34–36.
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS (2013a) Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 45: 1–14.
Jung KW, Won Y, Kong H, Oh C, Shin A, Lee J (2013b) Survival of Korean adult cancer patients by stage at diagnosis, 2006–2010: National Cancer Registry Study. Cancer Res Treat 45: 162–171.
Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX (2009) Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 15: 429–440.
Lordick F, Bang Y, Kang Y, Otero Reyes D, Manikhas G, Shen L, Kulikov E, Stoss O, Jordan B, Van Cutsem E (2007) HER2-positive advanced gastric cancer: similar HER2-positivity levels to breast cancer. Eur J Cancer 5: 272 (abstract 3541).
Malik MA, Totpal K, Balter I, Sliwkowski MX, Pelletier N, Reich M, Crocker L, Friess T, Bauer S, Fiebig HH, Allison DE (2003) Dose response studies of recombinant humanized monoclonal antibody 2C4 in tumor xenograft models. Proc Am Assoc Cancer Res 44: 150.
Matsuda T, Saika K (2013) The 5-year relative survival rate of stomach cancer in the USA, Europe and Japan. Jpn J Clin Oncol 43: 1157–1158.
Nahta R, Hung M, Esteva FJ (2004) The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 64: 2343–2346.
Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M (2009) Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 69: 9330–9336.
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai Y-, Ratnayake J, McNally V, Ross G, Cortés J (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24: 2278–2284.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire W (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER2-2/neu oncogene. Science 235: 177–182.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712.
Swain SM, Kim S, Cortés J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero J, Schneeweiss A, Knott A, Clark E, Ross G, Benyunes MC, Baselga J (2013) Pertuzumab, trastuzumab and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 14: 461–471.
Tanner M, Hollmen M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, Elenius K, Isola J (2005) Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 16: 273–278.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24: 2903–2909.
Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, Fujimoto-Ouchi K (2011) Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res 17: 5060–5070.
Yang J, Zhao H, Garnett C, Rahman A, Gobburu JV, Pierce W, Schechter G, Summers J, Keegan P, Booth B, Wang Y (2013) The combination of exposure-response and case-control analyses in regulatory decision making. J Clin Pharmacol 53: 160–166.
Zhang XL, Yang YS, Xu DP, Qu JH, Guo MZ, Gong Y, Huang J (2009) Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Surg 33: 2112–2118.
Zhu M, Doshi S, Gisleskog PO, Oliner KS, Perez Ruixo JJ, Loh E, Zhang Y (2014) Population pharmacokinetics of rilotumumab, a fully human monoclonal antibody against hepatocyte growth factor, in cancer patients. J Pharm Sci 103: 328–336.
Acknowledgements
The study was funded by F. Hoffmann-La Roche Ltd (Basel, Switzerland) and Genentech, Inc. (South San Francisco, CA, USA), a member of the Roche group. Targos Molecular Pathology (Kassel, Germany) performed central HER2 testing. Funding for third-party writing assistance was provided by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Y-KK and Y-JB have been advisory board members for and received honoraria from Roche. RY, TS and AG are employees of Genentech, a member of the Roche group. The remaining authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kang, YK., Rha, S., Tassone, P. et al. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer 111, 660–666 (2014). https://doi.org/10.1038/bjc.2014.356
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.356
Keywords
This article is cited by
-
HER2-targeted therapies — a role beyond breast cancer
Nature Reviews Clinical Oncology (2020)
-
Targeted Therapies in Advanced Gastric Cancer
Current Treatment Options in Oncology (2020)
-
Pertuzumab plus trastuzumab and chemotherapy for Japanese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subgroup analysis of the JACOB trial
International Journal of Clinical Oncology (2020)
-
Progress and challenges in HER2-positive gastroesophageal adenocarcinoma
Journal of Hematology & Oncology (2019)
-
Population pharmacokinetic and covariate analyses of intravenous trastuzumab (Herceptin®), a HER2-targeted monoclonal antibody, in patients with a variety of solid tumors
Cancer Chemotherapy and Pharmacology (2019)