Abstract
Background:
Pathological response (PR) to preoperative chemotherapy for colorectal liver metastases (CLM) is recognised as a prognostic factor of outcome. However, the optimal system to assess this parameter is still debated. This study focuses on current methods and proposes a possibly better method for assessing PR.
Methods:
Among 223 patients resected for CLM between 2004 and 2011, after more than three cycles of chemotherapy, the percentage of tumour cells, necrosis and fibrosis, and the tumour regression grade were assessed for each of 802 nodules. Pathological response was evaluated according to validated methods and their combinations. A new method combined the percentage of tumour cells and the size of all nodules as follows:  , where n is each separate nodule, % is the percentage of remaining tumour cells within nodule n (%) and s is the size of nodule n (cm).The prognostic value of each method was calculated.
, where n is each separate nodule, % is the percentage of remaining tumour cells within nodule n (%) and s is the size of nodule n (cm).The prognostic value of each method was calculated.
Results:
After a median follow-up of 47 months (3–106), the cumulative 5-year overall survival rate after liver resection was 59%. The proposed method categorised as follows: 0 residual tumour; 0.1–6-cm residual tumour; >6-cm residual tumour, and necrosis rate >50% stratified prognosis (P=0.0027; P=0.02), while the other methods did not. At multivariate analysis, our method remained an independent predictor of outcome (P=0.001).
Conclusions:
Combining the percentage of tumour cells multiplied by the size of each separate tumour seems to be a better method for assessing PR. External validation is required.
Similar content being viewed by others
Main
Preoperative systemic therapy and liver resection are widely used to treat patients with colorectal liver metastases (CLM). However, ∼70% of patients will relapse within 5 years (de Jong et al, 2009). Classical factors including carcinoembryonic antigen, maximal tumour size or tumour number are associated with recurrence and poor survival (Nordlinger et al, 1996), while some recent studies have questioned their prognostic value (Malik et al, 2007; Zakaria et al, 2007; Rees et al, 2008). There is increasing evidence that the pathological response (PR) to preoperative chemotherapy is a major determinant of outcome after resection, independently of others prognostics factors (Ribero et al, 2007; Blazer et al, 2008). Therefore, the assessment of the PR is of great clinical interest as it can help to select better postoperative chemotherapy.
To date, three main methods have been used to evaluate the PR—defined as an objective measurement of tumour cell viability. The two first reported methods were based on the percentage of residual tumour cells (Blazer et al, 2008) and the tumour regression grade (TRG) (Rubbia-Brandt et al, 2007), respectively. Both methods strongly differed in the assessment of the PR in case of multiple nodules with different individual responses. The method of Rubbia-Brandt et al (2007) accounted for the worse nodule, that is, the nodule with the highest TRG, while the method of Blazer et al (2008) defined the PR as the mean of the values for all the nodules in a given patient. Rubbia-Brandt et al (2007) and Blazer et al (2008) categorised patients into three tumour responses defined as: ‘major’ (TRG1 and TR2), ‘partial’ (TRG3) or ‘absent histological tumour’ response (TRG4 and TR5); and ‘complete’ (no residual tumour cells), ‘major’ (1–49% of residual tumour cells) and ‘minor’ (⩾50% of residual tumour cells) histological tumour response, respectively. In both methods, the distinction between the three categories of tumour responses has been of clinical relevance since they provided an immediate prognosis after resection that correlates with survival. A third method by Maru et al (2010) assessed the tumour cell viability by the maximum tumour thickness at the tumour–normal interface (TNI) for a nodule and the average tumour thickness for more than one nodule Maru et al (2010). The tumour thickness at the TNI correlated with the recurrence-free survival. Although tumour size is a predictor of major or complete PR, none of these methods accounted for this parameter in the assessment of PR (Rubbia-Brandt et al, 2007; Blazer et al, 2008; Brouquet et al, 2013; Vigano et al, 2013).
The different components of the PR other than the viable tumour cells (i.e., fibrosis, necrosis and acellular mucin) have been rarely studied. Response to chemotherapy corresponded either to an increase in the amount of necrosis (Loupakis et al, 2013) or a fibrotic involution of the nodules (Rubbia-Brandt et al, 2007; Poultsides et al, 2012). Fibrosis, but not necrosis predicted outcome (Poultsides et al, 2012). A fibrosis rate >40% even better correlated with the survival than the residual tumour cells.
To date, the optimal grading system to assess the PR remains a matter of debate (Gruenberger et al, 2012). To our knowledge, only one direct comparison of the methods for assessing PR based on the percentage of residual tumour cells has been made to date (Gruenberger et al, 2012). This recent study showed that the TRG was better than the percentage of tumour cells to predict patient prognosis. However, this study did not strictly use the original published methods in case of multiple nodules (Rubbia-Brandt et al, 2007; Blazer et al, 2008). The method by Maru et al (2010), based on the tumour thickness at the TNI , has been found to correlate with the PR assessed by the method of Blazer et al (2008).
The objectives of this study were to (1) implement and validate the current classifications for patients with CLM from a single institution and (2) propose a potentially better universal classification model for survival based on a single institution experience.
Patients and methods
Study population
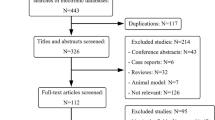
Between July 2004 and June 2011, 425 patients underwent elective liver resection for CLM at the Centre Hepato-biliaire, Paul Brousse Hospital, Villejuif, France. A selection was made to obtain a homogeneous group of patients. All patients who received at least three cycles of preoperative chemotherapy with no more than two lines of treatment and in whom all the tissue material was available for pathologic review were eligible for this study (Figure 1).
Patients without preoperative chemotherapy or less than three cycles, patients who received more than two lines of CT, patients who received bevacizumab and/or cetuximab in second line before liver resection, patients with unavailable tissue material, patients with operative mortality and patients with incomplete surgery (R2 resection) were excluded.
Pathological evaluation
The hepatectomy specimens were sectioned into 10 mm-thick (at a fresh status) and then into 5 mm-thick samples (after formalin fixation). Non-tumoral liver parenchyma distant from the tumours was routinely sampled. Presence (or absence) of chemotherapy-induced lesions went beyond the scope of this study and was not reviewed. The number of nodules, their size, usually measured from fresh tissue, and the margin status were recorded.
Nodules <2 cm were entirely sampled and bigger lesions were extensively sampled from the centre to the periphery. As recommended, one sample per centimetre along the greatest dimension was obtained. In patients with multiple nodules, each lesion was sampled.
Hematoxylin-eosin stained sections were reviewed by a single pathologist (MS), blinded to clinical information, treatment regimen and outcome. All parameters to implement known classifications were collected. In each CLM, the percentage of area with remaining viable tumour cells in relation to the total area of the CLM and its grade in TRG were evaluated. The percentage of tumour area showing coagulative necrosis and fibrosis was also noted.
Statistical analysis
Continuous data were expressed as median (range) and/or mean (standard deviation) whereas categorical data were expressed as percentage.
The analysis was first based on the PR per patient as continuous data (percentage of viable tumour cells). In patients with multiple nodules, the PR per patient was evaluated as the mean and the median of the values for all the nodules. The PR per patient was also evaluated as the mean and median of TRG values for all the nodules. The patients were then classified as having complete, major or minor response according to the categories of Blazer et al (2008), and major, partial or absent tumour response according to the categories of Rubbia-Brandt et al (2007), respectively.
Based on reported prognostic factors, a new classification was proposed where all tumours, the percentage of remaining tumour cells and the tumour sizes were combined as follows:  , where n is each separate nodule, % is the percentage of remaining tumour cells within nodule n (%) and s is the size of nodule n (cm).
, where n is each separate nodule, % is the percentage of remaining tumour cells within nodule n (%) and s is the size of nodule n (cm).
Other methods accounted for a single nodule possibly representing the overall PR. They included the nodule with the highest and lowest responses, and the closest nodule to the surgical section.
Subsequently, the percentage of coagulative necrosis and fibrosis of all the nodules was also analysed, adopting a cutoff of 50%.
The cumulative survival rate for each response group was calculated by the Kaplan–Meier survival curves and compared by log-rank test. Statistical significance was defined by a P-value <0.05. Statistical calculations were done with both R software (2.14.1; Free Software Foundation, Boston, MA, USA) and IBM SPSS Statistics 20 (IBM, Armonk, NY, USA).
Results
Clinical patient characteristics
The baseline characteristics of the study population are listed in Table 1. The cohort included 223 patients, 137 men and 86 women with a median age of 62 years (31–84 years). Thirty-seven per cent of the patients had initially unresectable hepatic disease. The diagnosis of CLM was made within 6 months of diagnosis of the primary tumour in 161 patients (72%). The primary tumour was located in the colon and the rectum in 80% (n=179) and 20% (n=44) of the patients, respectively. The primary tumour was graded T1/T2 in 30 patients (13%) and T3/T4 in 193 patients (87%). One hundred and sixty patients (71%) had regional node-positive primary colorectal carcinoma.
Of the study population, 120 patients (54%) received oxaliplatin-based chemotherapy, and 103 patients (45%) received irinotecan-based chemotherapy. Eighty-three patients (37%) received bevacizumab, and 36 patients (16%) received cetuximab. The median number of CT cycles was six (range, 3–34).
The median follow-up period was 47 months (range, 3–106 months). The cumulative 5-year overall survival rate was 59%.
Pathological features
Sixty-six patients (30%) had a single nodule. In the 167 patients with multiple nodules (70%), the number of nodules ranged from 2–32 per patient. The median and mean numbers of nodules were 3 and 3.66 nodules, respectively. A total of 802 nodules were reviewed. The median tumour size was 1.2 cm (range, 0.1–17 cm). One hundred and twenty-nine patients (58%) had positive resection margins for at least one nodule on pathological examination of the specimen.
A complete PR (no identifiable tumour cells in any nodule) was observed in 14 patients (6%). The mean and median PR were 31% and 25% (range, 0–100%), respectively.
The mean and median percentages of area occupied by coagulative necrosis were 27% and 20% (range, 0–100%), respectively, and for fibrosis, 33% and 30% (range, 0–100%), respectively.
Prognostic value of the methods for assessing PR
The PR was first analysed accounting for all the nodules in a given patient (Figure 2). Using the method by Blazer et al (2008) (i.e., PR per patient evaluated as the mean values for all the nodules), no survival differences between the three response curves were found (P=0.137, Figure 2 A). When the PR per patient was evaluated as the median values for all the nodules, the survival difference between the three response curves tended to reach statistical significance using the categories of Blazer et al (2008) (P=0.068, Figure 2C). However, there was no survival difference between ‘major’ and ‘minor’ responses. When implementing the modified method described by Rubbia-Brandt et al (2007) based on the mean and median TRG values, no differences between the three response curves were seen (P=0.127, Figure 2B; P=0.216, Figure 2D).
Kaplan–Meier survival curves according to the methods for the assessment of PR. (A) Mean percentage of remaining cancer cells according to the strict method of Blazer et al, (2008); (B) Mean TRG; (C) Median percentage of remaining cancer cells; (D) Median TRG; (E) Percentage of remaining cancer cells of the ‘worst’ nodule; (F) TRG of the ‘worst’ nodule according to the strict method of Rubbia-Brandt et al, (2007); (G) New proposed method 
By combining and categorising the features of all tumours ( ), there was a significant difference in overall survival (P=0.0027, Figure 2 G). There was also a significant difference between the curves using the cutoffs of 0, 6-cm residual tumour tissue and >6-cm residual tumour tissue when two groups were compared at a given time (P=0.055, P=0.006 and 0.005, respectively).
), there was a significant difference in overall survival (P=0.0027, Figure 2 G). There was also a significant difference between the curves using the cutoffs of 0, 6-cm residual tumour tissue and >6-cm residual tumour tissue when two groups were compared at a given time (P=0.055, P=0.006 and 0.005, respectively).
Subsequently methods based on a single nodule were analysed. The survival difference between the three response curves tended to reach statistical significance using the strict method described by Rubbia-Brandt et al (2007) when applied on the worse nodule (P=0.063, Figure 2F). However, there was no survival difference between ‘major’ and ‘partial’ responses. We obtained the same results by looking at the worse nodule using the cutoffs defined by Blazer et al (2008) (Figure 2E). We did not find any difference between the three response curves when the assessment was based on the nodule with the best response or the closest nodule to the cut surface.
When the degree of necrosis was assessed as the median value for all the nodules, we found a significant correlation with the overall survival (P=0.02, Figure 3A). In contrast, differences in the percentage of fibrosis did not affect the outcome (P=0.48, Figure 3B).
Univariate analysis (Table 2) revealed that, among the pathological variables, positive surgical margins did not predict survival. The PR assessed by  and a median percentage of necrosis >50% were associated with patient survival, as opposed to the methods described by Blazer et al (2008) and Rubbia-Brandt et al (2007). On multivariate analysis, the proposed method remained an independent predictor of outcome (P=0.001).
and a median percentage of necrosis >50% were associated with patient survival, as opposed to the methods described by Blazer et al (2008) and Rubbia-Brandt et al (2007). On multivariate analysis, the proposed method remained an independent predictor of outcome (P=0.001).
Discussion
This study focused on validating current classifications and proposes a potentially better method for assessing the tumour-based PR to chemotherapy in a large series of patients treated with preoperative chemotherapy for CLM. This study found that (1) the analysis of all the nodules using the strict method described by Blazer et al (2008) was not significantly discriminant with respect to patient survival and (2) the analysis of the nodule with the worse response using the strict method described by Rubbia-Brandt et al (2007) tended to reach statistical significance but was no more performant in this study population.
The PR to chemotherapy has been recognised as one of the strongest prognostic factor in patients treated with preoperative chemotherapy for CLM. However, the optimal grading system to assess this parameter is still debated (Gruenberger et al, 2012). Two main methods for assessing the PR based on the residual tumour cells have been recently reported (Rubbia-Brandt et al, 2007; Blazer et al, 2008). They provided a correlation of the PR with the overall survival. Even though disease-free survival might be desirable in survival analysis, this study also focused on the value of pathological review for the possible prediction of overall survival and is restricted to the available data. The interpretation of disease-free survival is controversial when it comes to disseminate cancers and accompanied by systemic treatments such as in the case of this study population (metastastic colorectal cancer).
Both published methods completely differed especially in case of multiple nodules. The method of Rubbia-Brandt et al (2007) is based on the worse nodule while Blazer et al (2008) uses the mean PR of all the nodules. Both classifications categorise patients into three groups: ‘major’ (TRG1 and 2), ‘partial’ (TRG3) and ‘absence of histological response (TRG4 and 5)’; and ‘complete’ (no residual tumour cells), ‘major’ (1–49% of residual tumour cells) and ‘minor’ (⩾50% of residual tumour cells) histological tumour response, respectively. It is important to note that the best category of tumour response slightly differs between classifications, for example, the best category according to Rubbia-Brandt et al (2007) includes complete (TRG1) and major response (TRG2), while the best tumour response according to Blazer et al (2008) only consists of complete response.
In the present study, the evaluation of the PR to chemotherapy was first made on all the nodules. When the method of Blazer et al (2008) was strictly applied to our study patients, using the mean value of responses, we did not observe a significant difference in the cumulative survival rate for each response group. By contrast, the modified method using the median rather the mean value had a better predictive value (P=0.068) but did not allow the distinction in terms of the overall survival between patients with ‘major’ and ‘minor’ responses. When the respective mean and median values of TRG were applied, there was no difference in the cumulative survival rates. This is in contrast with the recent study by Vigano et al (2013) which changed the strict definition of the method of Rubbia-Brandt et al (2007) using the median TRG rather than the TRG of the worst nodule. By also changing the method of Blazer et al (2008) using the median rather than the mean of the values for all nodules, this study showed that the (modified) TRG better stratified patient prognosis than the (modified) method of Blazer et al (2008).
Subsequently the evaluation of the PR to chemotherapy was based on the analysis of one nodule (i.e., the nodule with the worse and better response, and the closest nodule to the cut surface). The nodule with the worse response analysed according to either the categories of Rubbia-Brandt et al (2007) (corresponding to the strict method of Rubbia-Brandt et al (2007)) or the categories of Blazer et al (2008) tended to reach significance with respect to the overall survival in this study population (P=0.063 and 0.069, respectively). No differences between patients with ‘partial response’ and ‘absence of histological response’, or patients with ‘major’ and ‘minor’ responses were seen.
When implementing the classifications based on all the nodules (using the mean and median percentages of viable tumour cells, or the mean and median TRG), or the worse nodule (using the percentage of viable tumour cells or the TRG), no superiority between these methods was seen when applied to our patients. One explanation could be that the populations were not comparable in terms of the extent of the tumour disease and the type of surgical resections. Although the comparison is difficult to make, it seems that our patients displayed a higher median tumour size and a higher number of nodules than in previously reported series. This may be explained by a more aggressive attitude of the medico-surgical team for patients with multiple metastases and by the performance of multiple non-anatomical resections for parenchymal sparing surgery, leading to a higher proportion of resections with positive margins. However, positive margins did not emerge as a predictor of poor outcome. This is in line with a previous report by our group (de Haas et al, 2008), which suggested that the routine use of ultrasonic dissectors may spread tumour tissue to the peritumoral hepatic tissue, resulting in an overestimation of R1 resection rates. Moreover, potential remaining cancer cells may have been eradicated either by argon beam or coagulation of the cut section or by adjuvant chemotherapy.
By taking all these parameters into account, a new method was proposed that combined the PR and the tumour size (each separate nodule) is as follows:  This method significantly stratified patient prognosis in our population. The tumour size was preferred over tumour surface or volume to evaluate the residual tumour tissue for several reasons: (1) in order to calculate tumour surface or volume one must assume that the nodules are round and (2) surface and volume do not correspond to the sampling of lesions in case of nodules >2 cm. In practice, these nodules are not sampled in totality of their surface or volume, but along the greatest dimension.
This method significantly stratified patient prognosis in our population. The tumour size was preferred over tumour surface or volume to evaluate the residual tumour tissue for several reasons: (1) in order to calculate tumour surface or volume one must assume that the nodules are round and (2) surface and volume do not correspond to the sampling of lesions in case of nodules >2 cm. In practice, these nodules are not sampled in totality of their surface or volume, but along the greatest dimension.
Another objective measurement of tumour cell viability has been recently reported (Maru et al, 2010). The method was based on the maximum tumour thickness at the TNI for a nodule (and the average tumour thickness for more than one nodule). The tumour thickness at the TNI was measured perpendicular to the TNI (mm) at the focus with the maximum number of contiguous tumour cells. The authors explained that this method was simple as it is accompanied by an illustration showing the correct and incorrect method of measuring the maximum tumour thickness at the TNI for a round nodule. However, the nodules usually display irregular shapes, as illustrated by the dotted line in the photomicrograph of a nodule with poor response. It is not sure that the green arrow (showing the correct measurement) was perpendicular to the dotted line. This method excluded cases in which the entire tumour section was composed of tumour cells. In cases with a maximum tumour thickness of <0.5 mm, the measurement required ocular micrometre. Despite the difficulties in routine clinical practice, this method has been found to be reproducible (Brouquet et al, 2013). Because this method correlated with PR as assessed by the method of Blazer et al (2008) and disease-free survival, it will be important to warrant further investigations.
Few studies provided a detailed quantitative analysis of the components of the PR (i.e., fibrosis, necrosis and acellular mucin). In our study, mucin areas always accompanied with tumour cells (data not shown) were consequently assessed in the component of the remaining tumour cells. In two studies, response to chemotherapy corresponded to a fibrotic involution of the nodules without an associated increased in necrosis (Rubbia-Brandt et al, 2007; Poultsides et al, 2012). In the study by Poultsides et al (2012), fibrosis ⩾40% independently correlated with disease-free survival. The concept— necrosis is likely related to spontaneous phenomenon—is supported in the light of a significantly lower (Rubbia-Brandt et al, 2007) or similar (Poultsides et al, 2012) percentage of area occupied by necrosis in patients treated by preoperative chemotherapy compared with patients treated by surgery alone. We could not verify this hypothesis since all our included patients received preoperative chemotherapy. Furthermore, since the results of the EORTC trial (Nordlinger et al, 2008), the use of preoperative chemotherapy is now increasingly accepted to downsize tumours converting disease from unresectable to resectable and to identify responders and non-responders. Conversely, in the study by Loupakis et al (2013) and in the current study, the beneficial effects of chemotherapy appeared to be mainly related to the replacement of tumour by necrosis. In our study, the necrosis rate impacted on the outcome but less than the percentage of residual tumour cells weighted by the total tumour size (multivariate analysis).
In conclusion, PR based on analysis of the worse nodule using the strict method described by Rubbia-Brandt et al (2007) or all the nodules using the strict and the modified method by Blazer et al (2008) (i.e., mean and median of the responses, respectively) did not significantly predict the cumulative survival rate in our series. Combining the percentage of residual tumour cells multiplied by the size of the nodule of each separate tumour seemed to be the better method for assessing PR. This effect of PR on survival is mainly driven by high rates of necrosis. However, caution must be exercised before attempting to translate these results into clinical practice. Prospective and external validation is required.
Change history
29 July 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, Zorzi D, Ribero D, Ellis LM, Glover KY, Wolff RA, Curley SA, Abdalla EK, Vauthey JN (2008) Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 26: 5344–5351.
Brouquet A, Zimmitti G, Kopetz S, Stift J, Julie C, Lemaistre AI, Agarwal A, Patel V, Benoist S, Nordlinger B, Gandini A, Rivoire M, Stremitzer S, Gruenberger T, Vauthey JN, Maru DM (2013) Multicenter validation study of pathologic response and tumor thickness at the tumor-normal liver interface as independent predictors of disease-free survival after preoperative chemotherapy and surgery for colorectal liver metastases. Cancer 119: 2778–2788.
de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R (2008) R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg 248: 626–637.
de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM (2009) Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 250: 440–448.
Gruenberger T, Arnold D, Rubbia-Brandt L (2012) Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival. Surg Oncol 21: 309–315.
Loupakis F, Schirripa M, Caparello C, Funel N, Pollina L, Vasile E, Cremolini C, Salvatore L, Morvillo M, Antoniotti C, Marmorino F, Masi G, Falcone A (2013) Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br J Cancer 108: 2549–2556.
Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, Lodge JP, Toogood GJ (2007) Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg 246: 806–814.
Maru DM, Kopetz S, Boonsirikamchai P, Agarwal A, Chun YS, Wang H, Abdalla EK, Kaur H, Charnsangavej C, Vauthey JN, Loyer EM (2010) Tumor thickness at the tumor-normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol 34: 1287–1294.
Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D (1996) Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 77: 1254–1262.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371: 1007–1016.
Poultsides GA, Bao F, Servais EL, Hernandez-Boussard T, DeMatteo RP, Allen PJ, Fong Y, Kemeny NE, Saltz LB, Klimstra DS, Jarnagin WR, Shia J, D'Angelica MI (2012) Pathologic response to preoperative chemotherapy in colorectal liver metastases: fibrosis, not necrosis, predicts outcome. Ann Surg Oncol 19: 2797–2804.
Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247: 125–135.
Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, Chang DZ, Curley SA, Abdalla EK, Ellis LM, Vauthey JN (2007) Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 110: 2761–2767.
Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O, Chaussade S, Mentha G, Terris B (2007) Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol 18: 299–304.
Vigano L, Capussotti L, De Rosa G, De Saussure WO, Mentha G, Rubbia-Brandt L (2013) Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg 258: 731–740, discussion 741-2.
Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, Nagorney DM (2007) Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg 246: 183–191.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Sebagh, M., Allard, MA., Cunha, A. et al. A proposed new method for assessing the pathological response to chemotherapy in resected colorectal liver metastases. Br J Cancer 111, 470–476 (2014). https://doi.org/10.1038/bjc.2014.299
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.299
Keywords
This article is cited by
-
Long-term survival and cure model following liver resection for breast cancer metastases
Breast Cancer Research and Treatment (2018)