Abstract
Background:
This retrospective pooled analysis assessed the effect of age on the efficacy and safety of trabectedin in young and elderly patients with recurrent advanced soft tissue sarcoma (STS).
Methods:
Data from 350 adults with STS treated in five phase II trials with trabectedin were divided in the younger (<60 years; n=267) and the older cohort (⩾60 years; n=83).
Results:
The response rate did not differ with age (younger: 10.1% vs elderly 9.6%). No significant differences were found in median progression-free survival (PFS) in younger (2.5 months) and older (3.7 months) cohort with a comparable PFS rates at 3 (45.1% vs 55.1%) and 6 months (29.5% vs 36.4%). Similar median overall survival was observed in both cohorts (13.0 vs 14.0 months). Reversible neutropenia and aspartate aminotransferase/alanine aminotransferase elevation were the most common abnormalities. A higher incidence of grade 3/4 neutropenia (43.6% vs 60.2%) and fatigue (6.3% vs 14.4%) was observed in older patients. In 24 patients aged ⩾70 years, no significant differences in efficacy or safety outcomes were found.
Conclusion:
This analysis demonstrated that trabectedin is a feasible treatment in young and elderly patients with STS, with meaningful clinical benefits and an acceptable safety profile, essential in palliative treatment of elderly patients.
Similar content being viewed by others
Main
Trabectedin (Yondelis), is a synthetic antineoplastic drug originally isolated from the Caribbean sea squirt Ecteinascidia turbinate (Carter and Keam, 2010). Trabectedin binds covalently to the minor groove of the DNA double helix, stalling the replication fork and leading to double-strand breaks that triggers a cascade of events that ultimately leads in G2-M cell cycle arrest and apoptosis (D'Incalci and Galmarini, 2010). In addition to direct growth inhibition, trabectedin at therapeutic concentrations has selective anti-inflammatory and immunomodulatory properties because of the inhibition of production of factors that promote tumour growth, angiogenesis and metastasis (D’Incalci and Galmarini, 2010). Recent data also suggested that trabectedin selectively targets macrophages and downregulates the production of proinflammatory mediators, which induces changes in the tumour microenvironment contributing to its antitumour activity (Allavena et al, 2005; D'Incalci and Galmarini, 2010; Germano et al, 2010).
The efficacy of trabectedin as salvage chemotherapy in adults with advanced, recurrent soft tissue sarcoma (STS) has been assessed in three non-randomised phase II trials (Garcia-Carbonero et al, 2004; Yovine et al, 2004; Le Cesne et al, 2005) and in chemotherapy-naive patients with unresectable advanced STS (Garcia-Carbonero et al, 2005). A phase II randomised trial in advanced lipo- and leiomyosarcomas (L-sarcomas) after failure of prior conventional chemotherapy found a superior disease control of trabectedin 1.5 mg m–2 given as a 24-h intravenous (i.v.) infusion every 3 weeks (q3w) compared with a weekly trabectedin regimen (0.58 mg m–2; 3-h i.v. infusion for 3 consecutive weeks in a 4-week cycle; Demetri et al, 2009). It is noteworthy that the benefits from trabectedin therapy in patients treated with trabectedin given as a 24-h infusion q3w were highlighted by progression-free survival (PFS) rate at 3 months (51.5%) and 6 months (35.5%), which largely surpassed the thresholds criteria established by the European Organization for Research and Treatment of Cancer to define drug activity in pre-treated STS (i.e., 39% at 3 months and 14% at 6 months; Van Glabbeke et al, 2002). Based on these results, in 2007 trabectedin obtained marketing authorisation from the European Commission and in many other countries worldwide for the treatment of patients with advanced STS after failure of anthracyclines and ifosfamide, or for those patients who are unsuitable to receive these agents (European Medicines Agency (EMA), 2010).
Considering that nearly 50% of the patients with a newly diagnosed STS are over the age of 60 years at diagnosis, we performed a pooled analysis of data from five completed phase II trials (Garcia-Carbonero et al, 2004; Yovine et al, 2004; Garcia-Carbonero et al, 2005; Le Cesne et al, 2005; Demetri et al, 2009) to assess the age-related effects on the efficacy and safety of trabectedin.
Patients and methods
For this retrospective analysis, we have pooled all available data obtained in adult patients with STS treated in clinical trials with single-agent trabectedin at the approved dose and regimen: 1.5 mg m–2 given as a 24-h infusion q3w. We retrospectively analysed pooled data from 350 adult patients with STS treated in five phase II completed clinical trials. The efficacy and safety analysis of trabectedin in patients aged <60 and >60 years was performed by pooling individual data from 184 STS patients treated in three early non-randomised, single-arm, multicentre trials (Garcia-Carbonero et al, 2004; Yovine et al, 2004; Le Cesne et al, 2005), 36 chemotherapy-naive STS patients (Garcia-Carbonero et al, 2005) and 130 patients with L-sarcomas assigned to the 24-h q3w arm from the a pivotal, open-label, two-arm randomised trial (Demetri et al, 2009; Figure 1). In addition, a subset of 24 patients aged ⩾70 years was also analysed. All studies were conducted in European countries, the USA, Canada and Australia in accordance with the Declaration of Helsinki, guidelines for Good Clinical Practice and local regulations on clinical trials, and were approved by the institutional review boards of each participating centre. Signed informed consents were obtained from all study participants before registration.
All patients were required to have unresectable advanced or metastatic, histologically proven STS. Patients with refractory STS were allowed to receive combined or sequential prior chemotherapies and must have documented progressive disease (PD) less than or within 6 months of last treatment. Other eligibility criteria included patients ⩾18 years old, a minimum life expectancy of ⩾3 months, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ⩽1, advanced disease with at least one unidimensionally or bidimensionally measurable lesion, adequate renal, hepatic, and bone marrow function according to laboratory standard parameters, and recovery to National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade ⩽1 derived from any prior treatment-related toxicity. Exclusion criteria included brain or leptomeningeal involvement, prior exposure to chemotherapy or any experimental treatment concomitantly or 30 days before inclusion in the study, patients with a presence of other neoplastic diseases (with the exception of adequately treated non-melanoma skin carcinoma or carcinoma in situ), or any other serious or unstable medical or psychiatric condition.
An early phase II clinical trial demonstrated that co-medication with dexamethasone reduced drug-induced hepatotoxicity, without a deleterious effect on its anticancer activity (Paz-Ares et al, 2010). Accordingly, dexamethasone pre-treatment is considered mandatory for all patients receiving trabectedin. Additional antiemesis prophylaxis included ondansetron or granisetron, corticosteroids, and/or metoclopramide. Therapeutic use of either granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor or erythropoiesis-stimulating agents were allowed in accordance with American Society of Clinical Oncology guidelines (American Society of Clinical Oncology (ASCO), 1996).
Treatment could continue until PD or discontinuation for other reasons, such as unacceptable toxicity, investigator decision or consent withdrawal. Trabectedin was administered q3w provided the patient had completely recovered to baseline values from haematological and liver adverse events (AEs) and recovered to NCI-CTC grade ⩽1 from non-haematological AEs other than hepatic. Treatment could be delayed up to 2 weeks to allow recovery. If re-treatment criteria were not met by day 35, the patient was to be withdrawn from the study. A maximum of two dose reductions (from 1.5 to ∼1.2 mg m–2 then to 1.0 mg m–2) were permitted if any of the following events occurred during the previous cycle of therapy: grade 4 neutropenia longer than 5 days associated with fever; grade 4 thrombocytopenia; grade 2 cardiac or neurological toxicity; alkaline phosphatase or bilirubin increases of any grade; or any other grade 3/4 AEs other than grade 3/4 alanine aminotransferase or aspartate aminotransferase if it was reversed to baseline values by day 21.
The primary end point in all trials was to determine the overall response rate (ORR) as per investigator’s assessments or time-to-event end points, while safety was one secondary end point. Tumour response was assessed every two cycles according to the standard WHO criteria (Miller et al, 1981) or the Response Evaluation Criteria in Solid Tumors (Therasse et al, 2000), and PFS and OS curves were estimated by using the Kaplan–Meier method. The disease control rate (DCR) was defined as the percentage of patients with a complete response (CR) or partial response (PR) and/or stable disease (SD) lasting ⩾6 months. Eligible patients were considered assessable for response if they had received a minimum of two cycles of treatment and had at least one disease assessment performed at least 4 weeks after entering the study. Safety analyses were based on all-treated population, defined as those patients who received at least one trabectedin dose. Treatment-related AEs were coded using the Medical Dictionary for Regulatory Activities and graded according to the NCI-CTC.
Results
Patient characteristics
This analysis included 350 patients divided in two principal cohorts: the younger cohort included 267 (76%) patients aged <60 years (median age 48 years; range: 19−59 years) and the older cohort had 83 (24%) patients aged ⩾60 years (median age 65 years; range: 60−81 years). The number of patients with age ⩾60 years in five pooled studies ranged from 4.8% to 37.3%. Among the patients of the older cohort, a subset of 24 (7%) patients aged ⩾70 years (median age 73 years; range: 70−81 years) was separately analysed. Patients and tumour characteristics are summarised in Table 1. Patient and disease characteristics at baseline in either age-based group were similar and well balanced. In the younger and older cohort, L-sarcomas were the predominant histological STS subtypes (72% and 75%) and a good ECOG PS score of 0 out of 1 was recorded in 99.6% and 98.8% of patients, respectively. The vast majority of patients had previously undergone surgery (96% and 92%) and were pre-treated with a median of one line of chemotherapy (91% and 90%), respectively. Overall, most patients were exposed to one or two lines of prior chemotherapy (78.7% and 86.7%), respectively.
Treatment delivery
Median number of cycles received was 3, ranging from 1 to 48 and 1 to 59 cycles for younger and older patients, respectively. Patients received a median dose intensity of 0.42 mg m–2 per week over a median treatment duration of 10 weeks (range: 3.0–181.1) in the younger and 0.40 mg m–2 per week over a median treatment duration of 12 weeks (range: 3.0–236.7) in the older cohort (Table 2). In the younger and older cohort, 25.1% and 27.7% of patients received 7 or more cycles and 18.4% and 12.0% received 10 or more cycles, respectively, with a maximum of 48 and 59 cycles per cohort. Patients aged ⩾70 years had shorter median treatment duration (7.5 weeks; range: 3.0–37.3) mainly because of non-treatment-related events and received a median of two trabectedin cycles (range: 1−12). However, these patients reached a median dose intensity of 0.41 mg m–2 per week, representing 89% of the planned dose intensity, comparable with that given to patients younger than 70 years (Table 2).
Response rate and survival
Regarding the overall trabectedin activity, the ORR was 10.0% (2 CR and 33 PR; 95% confidence interval (CI): 7.1–13.6%) with no significant differences between patients aged <60 years (10.1%) and ⩾60 years (9.6%). A numerically lower overall ORR (1 PR, 4.2%; 95% CI: 1.1–21.1%) was observed among the 24 patients aged ⩾70 years. SD was recorded in 40.4%, 47.0% and 45.8% of patients aged <60, ⩾60 and ⩾70 years, respectively, and 17.2%, 21.7% and 20.8% of whom maintained SD for ⩾6 months for an DCR of 27.3%, 31.3% and 25.0% in each of these age-based groups (Table 3).
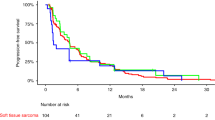
No significant differences were found in median PFS: 2.5 months in patients aged <60 vs 3.7 months in patients aged >60 (hazard ratio (HR): 0.9, 95% CI: 0.687–1.179; P=0.4427; Figure 2). Moreover, in both cohorts a comparable number of patients were progression-free at 3 months (45.1% vs 55.1%; P=0.115) and 6 months (29.5% vs 36.4%; P=0.2638). Similar median PFS was observed in ⩾70 years group (2.4 months; 95% CI: 1.4–6.2), as was the number of patients who were progression-free at 3 and 6 months: 45.8% (95% CI: 25.9–65.8%) and 32.1% (95% CI: 13.0–51.2%), respectively. Similarly, median OS was not statistically different in the two groups (13.0 vs 14.0 months in patients aged <60 and ⩾60 years, respectively; HR: 0.8, 95% CI: 0.61–1.06; P=0.1216), and comparable rates of OS were recorded in both cohorts: 55% and 56% at 12 months, and 29% and 38% at 24 months. No statistical differences (HR: 1.2, 95% CI: 0.76–1.86; P=0.4534) were observed in median OS in patients aged ⩾70 years (8.1 months; 95% CI: 4.6–19.4) and those aged <60 years (13.0 months; 95% CI: 11.3–14.9). Similarly, comparable number of patients older than 70 years was still alive at 12 months (41.7%, 95% CI: 21.9–61.4%).
Safety
Non-cumulative myelosuppression, with reversible neutropenia as the predominant component, and transient transaminase increases were the most common laboratory abnormalities seen with trabectedin associated to very low incidence of relevant clinical consequences. Treatment-related AEs outcomes are summarised in Table 4. Some grade 3/4 AEs were more common in patients aged >60 years, namely anaemia 10.1% vs 19.3%, neutropenia 43.6% vs 60.2%, thrombocytopenia 11.3% vs 20.5% and fatigue 6.4% vs 14.5%. Grade 3/4 neutropenia followed a predictable reversible pattern and was rarely associated with fever (one patient in each cohort). Major haematological complications were uncommon given that grade 3/4 febrile neutropenia occurred in 0.4% of patients aged <60 years and in 1.2% of patients aged >60 years, whereas the use of G-CSF was similar in both cohorts (12.7% vs 13.3%). Transaminase increases in both cohorts had a conventional self-limited pattern with a peak elevation within the first week of drug administration and returned to baseline values by days 10–15 of each cycle with a clear trend towards reduction with subsequent cycles. With the caveat of the small numbers of patients within the patient subset aged ⩾70 years, no major differences were found in the safety profile in this group with the lower use of G-CSF (4.2%) in this subset. Deaths associated with drug-related AEs were infrequent (1.9% and 2.4% of patients in the younger and older cohort, respectively). Overall, other AEs were infrequent and manageable (Table 4).
Discussion
This is the first analysis dealing with safety and efficacy in elderly patients with STS treated with trabectedin. The age of 70 is a chronological landmark commonly used in oncology for the definition of old patients (Pallis et al, 2010). However, this cutoff is arbitrary and the decision to treat or not these patients should be based on patients’ biological rather than the chronological age. Older patients may have an increased toxicity risk when treated with chemotherapy, mostly because of not treatment-related factors (Torosian et al, 1988). Considering that elderly sarcoma patients are often under-treated, this likely contributed to shorter sarcoma-specific survival rate (Lev and Pollock, 2010). Nijhuis et al (1999) reported that at least 50% of patients with metastatic sarcomas aged ⩾70 years were not treated at all and only 20% received chemotherapy, in contrast to the young patients (⩽20 years) who all received chemotherapy. Yet, some elderly patients may be over-treated, given that modest chemotherapy efficacy could be outweighed by its toxicity, thereby negatively affecting survival.
In the present pooled analysis, we considered that 60 years was a reasonable compromise between old age and the age of patients included in phase II trials. Our results show that trabectedin is an active treatment with an overall ORR of 10.0% (younger: 10.1% vs older: 9.6% patients). This ORR is comparable to that reported with low-dose ifosfamide (14%) and doxorubicin (13%) in pre-treated STS patients (Le Cesne et al, 2009). This benefit in both younger and older patient population was further confirmed by PFS rates at 3 months (45.1% vs 55.1%) and 6 months (29.5% vs 36.4%). Although in patients with metastatic STS, prolongation of survival may not be correlated with tumour response, high median OS was supportive for clinical activity of trabectedin being comparable in younger (13.0 months) and older (14.0 months) patients (Van Glabbeke et al, 1999). The results observed in the small and selected subset of 24 patients older than 70 years with excellent PS are encouraging; however, further exploration in a larger real-life population of elderly patients is warranted.
Beyond a direct and strong growth-inhibitory effect on cancer cells, trabectedin affects the tumour microenvironment by reducing the production of proinflammatory cytokines, such as IL-6, CCL2, CXCL8, VEGF and PTX3 (Germano et al, 2010). It is noteworthy that angiogenesis and immunosuppression in tumour biology frequently occur simultaneously in response to diverse stimuli being cross-regulated by overlapping pathways (Motz and Coukos, 2011). Therefore, the characteristic late and long-lasting responses reported with trabectedin now have gained better theoretical support under the standpoint of trabectedin not only as a cytotoxic but also as an immunomodulating drug with high anti-inflammatory and anti-angiogenic activity (Grosso et al, 2006, 2007).
The main AEs reported in all cohorts were reversible neutropenia and transaminase increases with no major differences in the safety profile between age groups. In agreement with the safety profile of trabectedin, the overall incidence and severity of those events decreased in frequency over cycles (Schoffski et al, 2007; Carter and Keam, 2010). As no cumulative toxicities were apparent in this analysis, trabectedin could be administered for prolonged periods (e.g., up to 59 cycles). This compares favourably with conventional treatment for STS, as doxorubicin-induced cumulative cardiotoxicity prevents protracted treatment and re-treatments in most cases (Ferreira et al, 2008), and renal toxicity and dose-limiting neutropenia have been largely associated with ifosfamide treatment (Le Cesne et al, 1995). Recently, in selected patients aged ⩽60 years the combination of doxorubicin and ifosfamide fail to significantly improve OS and was considerably more toxic than doxorubicin alone (van der Graaf et al, 2012).
In conclusion, the results of this pooled retrospective analysis have shown that trabectedin has an acceptable and manageable safety profile and antitumour activity in elderly patients with STS, comparable with that observed in overall population. No evidence of cumulative toxicity or end-organ dysfunction was found with trabectedin, and similar antitumour efficacy was recorded in all age-related groups after failure of conventional treatments or in patients who are not candidates to receive these drugs.
Change history
01 October 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Allavena P, Signorelli M, Chieppa M, Erba E, Bianchi G, Marchesi F, Olimpio CO, Bonardi C, Garbi A, Lissoni A, De Braud F, Jimeno J, D’Incalci M (2005) Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res 65 (7): 2964–2971.
American Society of Clinical Oncology (ASCO) (1996) Update of recommendations for use of hematopoetic colony-stimulating factors: evidence-based clinical practice guidelines. J Clin Oncol 14 (6): 1957–1960.
Carter NJ, Keam SJ (2010) Trabectedin: a review of its use in soft tissue sarcoma and ovarian cancer. Drugs 70 (3): 355–376.
D'Incalci M, Galmarini CM (2010) A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther 9 (8): 2157–2163.
Demetri GD, Chawla SP, von Mehren M, Ritch P, Baker LH, Blay JY, Hande KR, Keohan ML, Samuels BL, Schuetze S, Lebedinsky C, Elsayed YA, Izquierdo MA, Gomez J, Park YC, Le Cesne A (2009) Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol 27 (25): 4188–4196.
European Medicines Agency (EMA) (2010) Yondelis; trabectedin. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000773/human_med_001165.jsp&murl=menus/medicines/medicines.jsp&jsenabled=true . Accessed December 2010.
Ferreira AL, Matsubara LS, Matsubara BB (2008) Anthracycline-induced cardiotoxicity. Cardiovasc Hematol Agents Med Chem 6 (4): 278–281.
Garcia-Carbonero R, Supko JG, Maki RG, Manola J, Ryan DP, Harmon D, Puchalski TA, Goss G, Seiden MV, Waxman A, Quigley MT, Lopez T, Sancho MA, Jimeno J, Guzman C, Demetri GD (2005) Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol 23 (24): 5484–5492.
Garcia-Carbonero R, Supko JG, Manola J, Seiden MV, Harmon D, Ryan DP, Quigley MT, Merriam P, Canniff J, Goss G, Matulonis U, Maki RG, Lopez T, Puchalski TA, Sancho MA, Gomez J, Guzman C, Jimeno J, Demetri GD (2004) Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 22 (8): 1480–1490.
Germano G, Frapolli R, Simone M, Tavecchio M, Erba E, Pesce S, Pasqualini F, Grosso F, Sanfilippo R, Casali PG, Gronchi A, Virdis E, Tarantino E, Pilotti S, Greco A, Nebuloni M, Galmarini CM, Tercero JC, Mantovani A, D'Incalci M, Allavena P (2010) Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res 70 (6): 2235–2244.
Grosso F, Demetri GD, Blay JY, Judson I, Le Cesne A, Spreafico C, Jimeno J, Pilotti S, D'Incalci M, Casali PG (2006) Patterns of tumor response to trabectedin (ET743) in myxoid liposarcomas. J Clin Oncol 24 (18 Suppl): 9511.
Grosso F, Jones RL, Demetri GD, Judson IR, Blay JY, Le Cesne A, Sanfilippo R, Casieri P, Collini P, Dileo P, Spreafico C, Stacchiotti S, Tamborini E, Tercero JC, Jimeno J, D'Incalci M, Gronchi A, Fletcher JA, Pilotti S, Casali PG (2007) Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 8 (7): 595–602.
Le Cesne A, Antoine E, Spielmann M, Le Chevalier T, Brain E, Toussaint C, Janin N, Kayitalire L, Fontaine F, Genin J et al (1995) High-dose ifosfamide: circumvention of resistance to standard-dose ifosfamide in advanced soft tissue sarcomas. J Clin Oncol 13 (7): 1600–1608.
Le Cesne A, Blay JY, Judson I, Van Oosterom A, Verweij J, Radford J, Lorigan P, Rodenhuis S, Ray-Coquard I, Bonvalot S, Collin F, Jimeno J, Di Paola E, Van Glabbeke M, Nielsen OS (2005) Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol 23 (3): 576–584.
Le Cesne A, Domont J, Cioffi A, Bonvalot S, Terrier P, Ray-Coquard I, Alfaro V, Lebedinsky C, Santabarbara P, Blay JY (2009) Mapping the literature: role of trabectedin as a new chemotherapy option in advanced pretreated soft tissue sarcoma. Drugs Today (Barc) 45 (6): 403–421.
Lev D, Pollock RE (2010) Managing elderly soft tissue sarcoma patients—should age drive treatment? Ann Surg Oncol 17: 1725–1726.
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47 (1): 207–214.
Motz GT, Coukos G (2011) The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol 11 (10): 702–711.
Nijhuis PH, Schaapveld M, Otter R, Molenaar WM, van der Graaf WT, Hoekstra HJ (1999) Epidemiological aspects of soft tissue sarcomas (STS)—consequences for the design of clinical STS trials. Eur J Cancer 35 (12): 1705–1710.
Pallis AG, Fortpied C, Wedding U, Van Nes MC, Penninckx B, Ring A, Lacombe D, Monfardini S, Scalliet P, Wildiers H (2010) EORTC elderly task force position paper: approach to the older cancer patient. Eur J Cancer 46 (9): 1502–1513.
Paz-Ares L, Lopez-Pousa A, Poveda A, Balana C, Ciruelos E, Bellmunt J, Del Muro JG, Provencio M, Casado A, Rivera-Herrero F, Izquierdo MA, Nieto A, Tanovic A, Cortes-Funes H, Buesa JM (2010) Trabectedin in pre-treated patients with advanced or metastatic soft tissue sarcoma: a phase II study evaluating co-treatment with dexamethasone. Invest New Drugs 30 (2): 729–740.
Schoffski P, Wolter P, Clement P, Sciot R, De Wever I, Wozniak A, Stefan C, Dumez H (2007) Trabectedin (ET-743): evaluation of its use in advanced soft-tissue sarcoma. Future Oncol 3 (4): 381–392.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92 (3): 205–216.
Torosian MH, Friedrich C, Godbold J, Hajdu SI, Brennan F (1988) Soft-tissue sarcoma: initial characteristics and prognostic factors in patients with and without metastatic disease. Semin Surg Oncol 4: 13–19.
van der Graaf WTA, Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, Dei Tos AP, Marreaud S, Litiere S (2012) Results of a randomised phase III trial (EORTC 62012) of single agent doxorubicin versus doxorubicin plus ifosfamide as first line chemotherapy for patients with advanced or metastatic soft tissue sarcoma: a survival study by the EORTC Soft Tissue and Bone Sarcoma Group. Ann Oncol 23 (Suppl 9): Abstract LBA7.
Van Glabbeke M, van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, Verweij J, Santoro A, Buesa J, Tursz T, Blay JY, Le Cesne A, Judson I, Nielsen OS (1999) Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens-a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 17 (1): 150–157.
Van Glabbeke M, Verweij J, Judson I, Nielsen OS (2002) Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer 38 (4): 543–549.
Yovine A, Riofrio M, Blay JY, Brain E, Alexandre J, Kahatt C, Taamma A, Jimeno J, Martin C, Salhi Y, Cvitkovic E, Misset JL (2004) Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol 22 (5): 890–899.
Acknowledgements
This pooled analysis and all five completed phase II trials were supported by funding from PharmaMar, Madrid, Spain and Janssen Research and Development, LLC, Raritan, NJ 08869, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AL Cesne and J-Y Blay received research support, honoraria and travel grants from PharmaMar. A Nieto and A Tanović are employees and stockholders of PharmaMar, SA (Grupo Zeltia). S Schuetze received funding from Janssen to support the cost of conducting the trabectedin clinical trials. The remaining authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Cesne, A., Judson, I., Maki, R. et al. Trabectedin is a feasible treatment for soft tissue sarcoma patients regardless of patient age: a retrospective pooled analysis of five phase II trials. Br J Cancer 109, 1717–1724 (2013). https://doi.org/10.1038/bjc.2013.524
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.524
Keywords
This article is cited by
-
Systemic Treatment of Soft Tissue Sarcomas in the Geriatric Population
Current Treatment Options in Oncology (2022)
-
Treatment Strategy for Elderly Patients with Soft Tissue Sarcoma
Current Oncology Reports (2022)
-
Safety and efficacy of Pazopanib in advanced soft tissue sarcoma: PALETTE (EORTC 62072) subgroup analyses
BMC Cancer (2019)
-
Phase I study of the safety and pharmacokinetics of trabectedin with docetaxel in patients with advanced malignancies
Cancer Chemotherapy and Pharmacology (2015)
-
A Comprehensive Safety Evaluation of Trabectedin and Drug–Drug Interactions of Trabectedin-Based Combinations
BioDrugs (2014)