Abstract
Background:
Adrenocortical carcinoma (ACC) is a rare and aggressive endocrine malignancy without an available effective systemic chemotherapy. Insulin growth factor 2 (IGF-2) overexpression leading to the activation of the IGF-1 receptor (IGF-1R)/mammalian target of rapamycin (mTOR) pathway is well described in ACC. Cixutumumab, a fully human IgG1 monoclonal antibody directed at IGF-1R was combined with temsirolimus on the basis of preclinical data.
Methods:
Patients received cixutumumab, 3–6 mg kg−1 intravenously (IV) weekly, and temsirolimus, 25–37.5 mg IV weekly (4-week cycles), with restaging after 8 weeks.
Results:
Twenty-six patients were enrolled (13 (50%) men); median age, 47 years; median number of prior therapies, 4. Five patients previously received an IGF-1R inhibitor and one, temsirolimus. The most frequent toxicities, at least possibly drug related, were grade 1–2 thrombocytopenia (38%), mucositis (58%), hypercholesterolaemia (31%), hypertriglyceridemia (35%), and hyperglycaemia (31%). In all, 11 of 26 patients (42%) achieved stable disease (SD) >6 months (duration range=6–21 months) with 3 of the 11 having received a prior IGF-1R inhibitor.
Conclusion:
Cixutumumab combined with temsirolimus was well tolerated and >40% of patients achieved prolonged SD.
Similar content being viewed by others
Main
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy with an estimated annual prevalence of 0.5 to two cases per million population, and with many cases presenting as locally advanced or metastatic disease (Kebebew et al, 2006; Golden et al, 2009). Complete surgical resection, when feasible, is the optimal therapeutic strategy for improving survival (Lee et al, 1995; Grubbs et al, 2010). Despite best treatment efforts, patients with stage IV disease have a disease-specific survival at 5 years of only 13% (Fassnacht et al, 2009). The limited efficacy of standard-of-care therapy has led to the search for new treatment options.
Insulin growth factor 2 (IGF-2) is the single most upregulated transcript in as many as 80–90% of ACC cases. It is known to induce the activation of IGF-1 receptor (IGF-1R) and the insulin receptor (IR) (Fottner et al, 2004; Almeida et al, 2008; Barlaskar et al, 2009). During the dose escalation part of our phase I study with the IGF-1R inhibitor cixutumumab and the mammalian target of rapamycin (mTOR) inhibitor temsirolimus, antitumor activity was observed in metastatic ACC (Naing et al, 2011). Upstream tyrosine kinases such as IGF-1R regulate the PI3K/AKT/mTOR pathway (Liu et al, 2009). Furthermore, in vitro, in vivo, and tumour biopsy studies demonstrate that mTOR inhibitors activate a feedback loop, which results in upregulated AKT phosphorylation in tumour tissue via an IGF-1R-dependent mechanism (Hay and Sonenberg, 2004; Shi et al, 2005; O’Reilly et al, 2006; Wan et al, 2007). Pharmacologic inhibition of mTOR signalling by everolimus reduces ACC tumour cell lines growth in vitro and in vivo (Doghman et al, 2010).
The double targeting of the IGF1R-AKT-mTOR pathways by utilising two drugs that act at two different points in the pathways is a logical rationale. Hence, treatment with a combination of an IGF-1R inhibitor and an mTOR inhibitor would be a rational way to target ACC and circumvent resistance. Here, we report a cohort of 26 patients with ACC who were treated in an expansion cohort of our phase I study of the IGF-1R inhibitor cixutumumab and the mTOR inhibitor temsirolimus (Naing et al, 2011).
Materials and methods
Study design
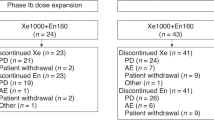
The patients reported herein were an expansion of a phase I, dose-expansion study that was conducted at The University of Texas MD Anderson Cancer Center and the Barbara Ann Karmanos Cancer Institute at Wayne State University (Naing et al, 2011). Twenty-six patients with ACC received cixutumumab, 6 mg kg−1 intravenously (IV) weekly, and temsirolimus, 25 mg (N=24) to 37.5 mg (N=2) IV weekly. Treatment cycles were 4 weeks with restaging after ∼8 weeks. This study was performed according to the principles embodied in the Declaration of Helsinki and after approval by the institutional review boards of both study centres. Informed consent was obtained from all patients enrolled on the study.
Toxicity
The severity of toxicity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0 initially and later changed to version 4.0 (per CTEP recommendation). Temperature, blood pressure, and pulse were measured before each infusion. Haematology, blood chemistry and urinalysis, and physical examinations were also monitored every week.
Evaluation of efficacy
Treatment efficacy was evaluated by computed tomography (CT) or magnetic resonance imaging (MRI) per Response Evaluation Criteria in Solid Tumours (RECIST) (Therasse et al, 2000) before treatment and approximately every 8 weeks thereafter. Briefly, a complete response (CR) was disappearance of all lesions, partial response (PR) was a ⩾30% reduction in the sum of the longest diameters of the lesions, stable disease (SD) was denoted in patients whose sum of longest lesion diameters were not decreased >30% and not increased >20%, and progressive disease (PD) was a ⩾20% increase in the sum of the longest diameters of the lesions. A response had to last for at least 4 weeks to be considered as a PR or CR. Patients with SD lasting 6 months or longer were considered to have durable SD.
Results
Patient characteristics
A total of 26 patients (13 men) with advanced metastatic and/or refractory ACC were enrolled on the study. Median age of participants was 47 years (range, 20–74 years). All pathologic diagnoses were confirmed at MD Anderson or Wayne State University. The number of tumour organs involved at study entry for all 26 patients is 1–4, and the most common site is lung. Ten out of twenty-six patients were documented to have secreting ACC. Three patients received prior IGF-1R inhibitor treatment, one patient was on a randomised trial and received either placebo or IGF-1R inhibitor treatment, and one patient had been previously treated with temsirolimus. Most patients had been heavily pretreated, with the median number of prior therapies being 4 (range 0–8).
Toxicities
The current study represents an expansion of a previous phase I dose escalation study (Naing et al, 2011). The 26 patients reported in this study with ACC had the following toxicities, that were at least possibly drug-related at both dose levels, but most instances of them were grade 1 or 2 (Table 1): mucositis (n=15), thrombocytopenia (n=10), hypertriglyceridemia (n=9), hypercholesterolaemia (n=8), and hyperglycaemia (n=7). Hyperglycaemia was managed in collaboration with an endocrinologist. Two patients who were diabetic at baseline were controlled by insulin and sitagliptin or by glipizide. Two of the rest of the twenty-four study patients developed diabetes mellitus on study, which was well controlled with the use of metformin alone (n=1), and insulin together with metformin and glipizide (n=1).
We have previously reported toxicities in the heterogeneous tumour type population as well as the Ewing’s sarcoma family tumours treated with this drug combination (Naing et al, 2011, 2012). Compared with the patients with heterogeneous tumour types and Ewing’s sarcoma family tumours, there were no peculiar toxicities that were unique to ACC patients. We did not observe a difference of toxicity for patients who had secreting ACC.
Antitumor activity
Tumour response was assessed by RECIST criteria (Therasse et al, 2000). The best responses for the 26 study patients are shown in the waterfall plot in Figure 1. In all, 11 of 26 patients (42%) had SD ⩾6 months. Three out of the eleven responders were documented to have secreting ACC. Two patients whose tumours remained stable for at least 8 months had prior IGF-1R inhibitor treatment.
3D RECIST Waterfall plot. Best response by RECIST in 26 treated patients with ACC. Dose level 3 (N=24) was cixutumumab, 6 mg kg−1 IV weekly, and temsirolimus, 25 mg IV weekly. Dose level 4 (N=2) was cixutumumab, 6 mg kg−1 IV weekly, and temsirolimus, 37.5 mg IV weekly. Patients with early clinical progression or with new lesions or clinical progression or who came off early for any reason are indicated on the graph as a 21% increase (*).
Discussion
Many patients with ACC present with locally advanced or metastatic disease (Kebebew et al, 2006; Golden et al, 2009). Eighty percent of patients have disease recurrence within 2 years after curative surgery, and common sites of metastasis are liver, lung, and adjacent organs. In patients with recurrent ACC, disease-free survival after curative surgery is 12.1 months (Luton et al, 1990; Wooten and King, 1993). Unfortunately, the available systemic therapeutic options do not consistently result in effective cytoreduction. The limited efficacy of available systemic chemotherapy led to a search for new treatment options based on the underlying molecular mechanisms involved in ACC.
Insulin growth factor 2 (IGF-2) is upregulated in ACC; and IGF-2 signalling is mediated through its interaction with the IGF-1R, which leads to downstream activation of mTOR (Pavelic et al, 2002; Demeure et al, 2011). Cixutumumab is a fully human monoclonal antibody that inhibits IGF-1R. Preclinical in vitro and animal studies showed reduced ACC cell proliferation induced by cixutumumab that was augmented in combination with the antineoplastic agent mitotane (Barlaskar et al, 2009). During our dose escalation study, 4 out of 10 ACC patients had SD over 8 months (Naing et al, 2011). The dose expansion phase was then done in an additional 16 patients. As reported here, 11 out of a total of 26 patients (42%) had durable (⩾6 months) SD. There were, however, no PR or CRs in the study patients. In the 11 patients who had SD over 6 months, the median time to progression (TTP) on combination of temsirolimus and cixutumumab was 9 months compared with 4 months of median TTP on their previous treatment regimen.
Recent preclinical study showed that sirolimus inhibits cortisol secretion in ACC (De Martino et al, 2012). In this study, 10 out of 26 patients were documented to have secreting ACC, and hormonal levels were not analysed throughout the study. One responder and one non-responder were managed by board-certified endocrinologists for hormonal-related symptoms; therefore, it is unclear whether this combination of temsirolimus and cixutumumab affected the hormone levels or improved hormonal-related symptoms in these patients with ACC.
Temsirolimus is metabolised by the microsomal liver enzyme cytochrome P450 (CYP3A4/5). Drugs interfering with these enzymes were suspended 4 weeks before starting the protocol treatment (Naing et al, 2011). Mitotane is the most commonly used drug for the treatment of ACC and can induce this enzyme, and it may cause sub-therapeutic levels of temsirolimus.
Most of our patients had been heavily pretreated, with the median number of prior regimens being 4. Ten out of twenty-six patients received mitotane as their most recent treatment before enrolling in our study. Ideally, mitotane plasma levels should be <5 μg ml−1 at baseline; however, in our study, the mitotane levels in each patient were not recorded. Four patients had received prior treatment with an IGF-1R inhibitor (n=3) or mTOR inhibitor (n=1) and one patient had previously been on a randomised, blinded trial and received either placebo or an IGF-1R inhibitor. Prior use of and progression on a single agent IGF-1R inhibitor did not preclude a prolonged SD response to the combination of an IGF-1R inhibitor and an mTOR inhibitor. Two of three patients with prior IGF-1R inhibitor treatment achieved durable (⩾6 months) SD. One patient was a 34-year-old woman who was treated with a single-agent IGF-1R inhibitor for nearly 10 months. Upon progression, she was enrolled on our trial and remained stable for 10 months. The second patient’s tumour remained stable on our combination treatment trial of temsirolimus and cixutumumab for 9 months. There was also a third patient who was a 62-year-old woman who had previously been treated with either placebo vs IGF-1R inhibitor for 3 months before being enrolled on our study. The patient’s tumour remained stable for 8 months.
Overall, this regimen was well tolerated. Side effects were manageable and patients continued to maintain their performance status until their disease progressed. Endocrine complications such as hyperglycaemia and hyperlipidemia were observed. This was not unexpected since a concern for this class of drugs, especially the IGF-1R inhibitors, is that they induce hyperglycaemia (Haluska et al, 2010). As a result, many studies restrict eligibility so that patients with elevated blood glucose cannot enrol. This study was not restricted in this way. We found that hyperglycaemia was managed with oral hypoglycaemia agents, with or without insulin, and patients (N=2) who were diabetic at baseline did not worsen. Only two patients who were not diabetic at baseline became diabetic on study, and they were managed with metformin alone (n=1) or oral hypoglycaemia agents and insulin (n=1). The second patient on oral hypoglycaemia agents and insulin remained stable for 12 months. It is not clear if his diabetes was reversible after discontinuation of study drugs, as he returned home to a foreign country after 12 months, and was lost to follow-up. These results, along with those from our previous study demonstrated that patients who develop metabolic side effects such as hyperglycaemia or more significant myelosuppression during the study may have superior responses. Furthermore, those who develop worsening hyperglycaemia should be treated for high blood sugar rather than removed from the trial (Naing et al, 2012).
Treatment of ACC remains challenging and the efficacy of current therapies such as mitotane and etoposide has been dismal. First-line treatment on a combination of etoposide, doxorubicin, and cisplatin (EDP) with mitotane produced a better rate of response and progression-free survival compared with streptomycin plus mitotane, however, overall survival remained disappointing at <15 months (Fassnacht et al, 2012). Various targets and agents have been explored in ACC (Almeida et al, 2008; Demeure et al, 2011). The epithelial growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib did not show efficacy as a single agent in ACC (Samnotra et al, 2007). Similarly, sunitinib exhibited modest activity as a single agent in mitotane-exposed ACC patients (Kroiss et al, 2012). Other pathways such as those involving fibroblast growth factor receptor (FGFR) and Wnt-β-catenin signalling cascades and loss of p53 function have been implicated in ACC tumorigenesis. The development of strategies targeting these pathways may also be worthwhile (Demeure et al, 2011; Simon and Hammer, 2012). This trial shows promise in the management of refractory ACC. However, without conducting a randomised phase II/III trial, it is unclear whether this treatment regimen would be more effective than second-line or third-line standard therapy.
The combination of cixutumumab and temsirolimus demonstrates modest activity in refractory ACC as well is in other types of cancer such as Ewing’s sarcoma and desmoplastic small-round-cell tumours (DSRCT) (Naing et al, 2012). Our patients with ACC were heavily pretreated and future studies in larger numbers of patients with ACC are needed to better evaluate the benefit of this treatment, perhaps earlier in the course of their disease.
Change history
05 March 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH, Lerario AM, Maciel CC, Mattos GE, Jorge AA, Mendonca BB, Latronico AC (2008) Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab 93 (9): 3524–3531
Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD (2009) Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab 94 (1): 204–212
De Martino MC, van Koetsveld PM, Feelders RA, Sprij-Mooij D, Waaijers M, Lamberts SW, de Herder WW, Colao A, Pivonello R, Hofland LJ (2012) The role of mTOR inhibitors in the inhibition of growth and cortisol secretion in human adrenocortical carcinoma cells. Endocr Relat Cancer 19 (3): 351–364
Demeure MJ, Bussey KJ, Kirschner LS (2011) Targeted therapies for adrenocortical carcinoma: IGF and beyond. Horm Cancer 2 (6): 385–392
Doghman M, El Wakil A, Cardinaud B, Thomas E, Wang J, Zhao W, Peralta-Del Valle MH, Figueiredo BC, Zambetti GP, Lalli E (2010) Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res 70 (11): 4666–4675
Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B German Adrenocortical Carcinoma Registry Group; European Network for the Study of Adrenal Tumors (2009) Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer 115 (2): 243–250
Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardière C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Müller HH, Skogseid B FIRM-ACT Study Group (2012) Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 366 (23): 2189–2197
Fottner Ch, Hoeflich A, Wolf E, Weber MM (2004) Role of the insulin-like growth factor system in adrenocortical growth control and carcinogenesis. Horm Metab Res 36 (6): 397–405
Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW (2009) Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab 94 (6): 1853–1878
Grubbs EG, Callender GG, Xing Y, Perrier ND, Evans DB, Phan AT, Lee JE (2010) Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol 17 (1): 263–270
Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A, Hammer GD (2010) Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol 65 (4): 765–773
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18 (16): 1926–1945
Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A (2006) Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg 30 (5): 872–878
Kroiss M, Quinkler M, Johanssen S, van Erp NP, Lankheet N, Pöllinger A, Laubner K, Strasburger CJ, Hahner S, Müller HH, Allolio B, Fassnacht M (2012) Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab 97 (10): 3495–3503
Lee JE, Berger DH, el-Naggar AK, Hickey RC, Vassilopoulou-Sellin R, Gagel RF, Burgess MA, Evans DB (1995) Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery 118 (6): 1090–1098
Liu P, Cheng H, Roberts TM, Zhao JJ (2009) Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 8 (8): 627–644
Luton JP, Cerdas S, Billaud L, Thomas G, Guilhaume B, Bertagna X, Laudat MH, Louvel A, Chapuis Y, Blondeau P, Bonnin A, Bricaire H (1990) Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med 322 (17): 1195–1201
Naing A, Kurzrock R, Burger A, Gupta S, Lei X, Busaidy N, Hong D, Chen HX, Doyle LA, Heilbrun LK, Rohren E, Ng C, Chandhasin C, LoRusso P (2011) Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res 17 (18): 6052–6060
Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, Ludwig J, Chen HX, Doyle LA, Kurzrock R (2012) Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin Cancer Res 18 (9): 2625–2631
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66 (3): 1500–1508
Pavelic K, Buković D, Pavelić J (2002) The role of insulin-like growth factor 2 and its receptors in human tumors. Mol Med 8 (12): 771–780
Samnotra V, Vassilopoulou-Sellin R, Fojo AT, Oh WK, LaRocca RV, Ernstoff MS, Memoli VA, Cole BF, Quinn DI, Simmons PA, Tretter CP (2007) A phase II trial of gefitinib monotherapy in patients with unresectable adrenocortical carcinoma (ACC). J Clin Oncol 25, (abstract 15527)
Shi Y, Yan H, Frost P, Gera J, Lichtenstein A (2005) Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther 4 (10): 1533–1540
Simon DP, Hammer GD (2012) Adrenocortical stem and progenitor cells: implications for adrenocortical carcinoma. Mol Cell Endocrinol 351 (1): 2–11
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92 (3): 205–216
Wan X, Harkavy B, Shen N, Grohar P, Helman LJ (2007) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26 (13): 1932–1940
Wooten MD, King DK (1993) Adrenal cortical carcinoma. Epidemiology and treatment with mitotane and a review of the literature. Cancer 72 (11): 3145–3155
Acknowledgements
We acknowledge Kristie Lawhorn, RN, for coordinating and data collection, and Joann Aaron, MA, for scientific review of and editing the paper. This study was supported by R21CA13763301A1 (AN), U01CA62461 (RK), and U01CA62487 (PL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
There authors declare no conflict of interest.
Additional information
Presented in part at the American Association for Cancer Research-National Cancer Institute-European Organization for Research and Treatment of Cancer Molecular Targets and Cancer Therapeutics, 15–19 November 2009, Boston, MA; the National Cancer Institute/Cancer Therapy Evaluation Program Early Drug Development Meeting, October 2009, Bethesda, MD; the National Cancer Institute/Cancer Therapy Evaluation Program Early Drug Development Meeting, October 2011, Bethesda, MD; the 46th Annual Meeting of the American Society of Clinical Oncology, 4–8 June 2010, Chicago, IL; the 47th Annual Meeting of the American Society of Clinical Oncology, 3–7 June 2011, Chicago, IL.
Précis
The combination of cixutumumab and temsirolimus was well tolerated in patients with rare and aggressive ACC. More than 40% of ACC patients achieved prolonged SD using this combination.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Naing, A., LoRusso, P., Fu, S. et al. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer 108, 826–830 (2013). https://doi.org/10.1038/bjc.2013.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.46
Keywords
This article is cited by
-
IGF and mTOR pathway expression and in vitro effects of linsitinib and mTOR inhibitors in adrenocortical cancer
Endocrine (2019)
-
Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial
Journal for ImmunoTherapy of Cancer (2018)
-
Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors
Nature Communications (2018)
-
IGF-IR signaling in epithelial to mesenchymal transition and targeting IGF-IR therapy: overview and new insights
Molecular Cancer (2017)
-
5th International ACC Symposium: Future and Current Therapeutic Trials in Adrenocortical Carcinoma
Hormones and Cancer (2016)