Abstract
Background:
Herpes zoster and cancer are associated with immunosuppression. Zoster occurs more often in patients with an established cancer diagnosis. Current evidence suggests some risk of cancer after zoster but is inconclusive. We aimed to assess the risk of cancer following zoster and the impact of prior zoster on cancer survival.
Methods:
A primary care database retrospective cohort study was undertaken. Subjects with zoster were matched to patients without zoster. Risk of cancer following zoster was assessed by generating hazard ratios using Cox regression. Time to cancer was generated from the index date of zoster diagnosis.
Results:
In total, 2054 cancers were identified in 74 029 patients (13 428 zoster, 60 601 matches). The hazard ratio for cancer diagnosis after zoster was 2.42 (95% confidence interval 2.21, 2.66) and the median time to cancer diagnosis was 815 days. Hazard ratios varied between cancers, and were highest in younger patients. There were more cancers in patients with zoster than those without for all age groups and both genders. Prior immunosuppression was not associated with change in risk, and diagnosis of zoster before cancer did not affect survival.
Conclusion:
This study establishes an association between zoster and future diagnosis of cancer having implications for cancer case finding after zoster diagnosis.
Similar content being viewed by others
Main
Herpes zoster (shingles) is the reactivation of latent varicella zoster virus, and has an incidence ranging from 1.2 to 4.8 per 1000, increasing markedly with age (Thomas and Hall, 2004). It is associated with immunosuppression, both genetic and acquired, and has been strongly associated with a known diagnosis of cancer, a future diagnosis of HIV/AIDS, (Arvin, 1996; Thomas and Hall, 2004), and stroke (Kang et al, 2009).
The possibility that zoster might presage a diagnosis of cancer was first suggested in 1955 (Wyburn-Mason, 1955). Subsequent studies have failed to fully establish this association (Ragozzini et al, 1982; Fueyo and Lookingbill, 1984; Zaha et al, 1993; Yamamoto et al, 2003). Three larger and more recent studies have suggested that there may be an association. First, Sørensen et al (2004) reported a relative risk for all cancer types for zoster as 1.2 and suggested that screening for cancer would have ‘low efficacy’. However, this study was of patients hospitalised for zoster (a minority of zoster cases, and those with more severe disease), rather than those diagnosed and managed in the community. Buntinx et al (2005), from a primary care database study, failed to demonstrate any increase in cancer diagnoses within a year of zoster in those under 65; however, this study did report a significant difference in females over 65 years (HR 2.65). Ho et al (2011) from a Health Insurance Research Database in Taiwan reported that the risk of cancer after herpes zoster ophthalmicus was 9.25 times greater than their matched comparison cohort. Additionally, patients with zoster had lower 1 year cancer-free survival than their comparison cohort. A more recent study from Taiwan showed no increased risk of cancer after zoster compared with expected incidence rates (Wang et al, 2012).
It is therefore not clear whether there is an association between a previous diagnosis of zoster (in an unselected primary care population) and subsequent diagnosis of cancer. Determining the risk of cancer following zoster is important because it raises the potential for case finding to facilitate earlier cancer diagnosis. The primary aim of this study was to determine the risk of a diagnosis of any primary cancer following zoster compared with patients without zoster, using a database of a large primary care cohort of patients. Secondary aims were to assess if specific cancers were associated with a previous diagnosis of zoster, and to determine the difference in survival in patients with cancer in the data set between those who had a prior diagnosis of zoster and those who did not.
Materials and methods
Patient selection and matching
The General Practice Research Database (GPRD) (now renamed as CPRD) is a large UK primary care database containing the general practice information of over 3.6 million patients from >450 practices. The information available is the medical, practice nursing, and administration records of individual patients, which includes clinical diagnoses, investigation results, prescriptions, referral decisions, and referral outcomes.
The baseline population consisted of all ‘up-to-standard’ patients in the GPRD of 1 January 1987 to 12 December 2002 inclusive. ‘Up-to-standard’ is a marker that indicates when data recording by the patient’s practice meet the specific quality measures defined by the UK Medicines and Healthcare products Regulatory Agency (MHRA) (CPRD, 2012).
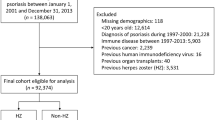
Cases (n=13 428) were all patients 18 years or older with a recorded medical diagnosis of zoster during the period 1 January 2001 to 12 December 2002 inclusive. A medical diagnosis of zoster was defined using a list of predefined Read/OXMIS codes. Cases were matched with patients without a prior diagnosis of zoster by gender and year of birth, in a ratio of 1 : 4.5, as recommended by Smeeth et al (2006). Both cases and the matched subjects had >5 years of GPRD records before their date of zoster diagnosis (cases) or pseudo-diagnosis (for matches without zoster) and no documented diagnosis of cancer (excluding non-melanoma skin cancer) during this period. We chose this time period for pragmatic reasons, although it was a trade-off against trying to find relevant past exposure of cancer and immunosuppression (see later), while trying to maximise the numbers of cases and controls, especially for the survival analysis. All records in the data set were followed up until a diagnosis of any primary cancer or for a maximum of 5 years from the respective date of zoster diagnosis or pseudo-diagnosis. Records for cases and non-cases were evaluated for first recorded cancer diagnosis (excluding non-melanoma skin cancer, and whatever the level of spread at the time of diagnosis) after the zoster diagnosis. These records were then categorised for type of cancer.
Power calculation
By comparing two groups using Cox regression, the sample size can be obtained from the formula for the log-rank test given in Hsieh et al (2003). Buntinx et al (2005) found that the number of patients without cancer was 97.94% and 99.26% for the zoster and no zoster groups, respectively; this yields an estimated hazard ratio (h) of 2.7. Using this figure, the proportion of controls, a power of β=0.9 and a significance level of α=0.05, STATA 10 gave E=43, N1=3573, and N2=785 where E is the estimated number of cancer events, N1 and N2 are the required sample sizes for the unexposed/exposed groups, respectively. Thus, it can be seen that the study has more than sufficient number of subjects.
Analysis
The Cox proportional hazards model was used to compare the subsequent risk of cancer in patients with and without zoster. Using the index date of the matched case, the time to cancer diagnosis was generated. Subjects who did not develop cancer after 5 years of follow-up were censored at this time. To test the possibility of there being cancers diagnosed just before a diagnosis of zoster that had not been recorded in the data set until afterwards, a sensitivity analysis excluding all cancers diagnosed within 2 months of a diagnosis of zoster was done (the time duration was based upon clinical common sense; we envisaged that this would be the likely time that a pre-existing cancer would have been diagnosed within, especially as the undiagnosed cancer was responsible for the zoster). Furthermore, because the risk of zoster and cancer vary with age, this model allowed gender-stratified and age-adjusted analyses. The Cox regression was routinely assessed using Schoenfield’s residual test to test the fit of the proportional hazards model (Grambsch and Therneau, 1994). To contextualise the meaning of the hazard ratios into clinical practice, we summarised the absolute numbers of cancers diagnosed within different time durations after zoster diagnosis (Table 2).
The analysis was repeated to assess whether patients exposed to immunosuppression (which may increase the risk of both conditions) changed the overall zoster hazard ratio. Subjects with and without zoster were classed as immunosuppressed if they met one of the following criteria shown in Box 1 before their cancer diagnosis. This was based upon the definition from that used by Salisbury et al (2006), which identifies potential severe immunosuppression such that such patients should not receive live vaccines. It was only modified to exclude malignant disease and its treatment (because these patients have been excluded already).
To fulfill the second aim to estimate the difference in survival, patients with cancer were matched where possible (by age within 5 years, gender, and cancer site) in a 1 : 1 ratio dependent upon whether they had a zoster diagnosis or not. Cases without matches were excluded. Analysis was then performed using Kaplan–Meier estimates of the time to event.
SPSS version 16 was used for handling the data and statistical analysis (SPSS Inc., Chicago, IL, USA).
Results
In total, 74 029 patients were studied; 13 428 patients with zoster were matched with 60 601 patients without zoster (>1 : 4.5). The range of follow-up for both groups was 1–1825 days. The mean age of patients with zoster was 59.6 years and without zoster was 60.0 years. There were 42% males in each group. During the study period, a total of 2054 patients developed a primary cancer. Of these, 658 developed in patients with zoster and 1396 developed in patients without zoster.
Table 1 summarises the hazard ratios for patients developing cancer after a diagnosis of zoster for all cancers and by cancer site. Median times from zoster diagnosis to cancer diagnosis are also shown. For all cancers combined, the hazard ratio was 2.42 (95% confidence interval (CI) 2.21, 2.66) and the median time from zoster to cancer was 815 days. Ovarian cancer had the highest hazard ratio of 5.35 (95% CI 2.85, 10.03) and connective tissue cancers the shortest median time to cancer (657 days – interquartile range (IQR) 382, 1664). Only a small number of cancer groups (bladder, miscellaneous, hepatocellular/bile duct, and stomach) did not have hazard ratios that were significant at the 95% CI.
Repeating the analysis but excluding all cancers diagnosed within 2 months of a diagnosis of zoster reduced the hazard ratio of a patient developing cancer slightly to 2.32 (95% CI 2.11, 2.55). The median time to diagnosis was 1723 days (IQR 1460, 1800).
Of the 2054 patients with cancer, 996 were male and 1058 were female. The hazard ratio of cancer in zoster vs non-zoster was highest for the youngest age band (18–50 years); HR of 6.57 (95% CI 4.28, 10.41); however, the numbers of cancers were smaller with 2.73 per 1000 person years for patients with zoster and 0.45 per 1000 person years for those without zoster. The risk was less for older age bands.
Residual diagnostic tests suggested that the proportional hazard assumptions were not met for some time points (Table 2). An extended Cox model using an interaction term of time and zoster exposure was introduced to take into account the effect of drop out and follow-up time. The difference between the zoster and no zoster groups was most marked soon after zoster. After 12 months, there were proportionately four times as many cancers in the zoster group. By 5 years, almost 10% of men over 65 years who had zoster had developed cancer. The hazard ratios are highest soon after diagnosis, but the CIs are wide, reflecting the limited number of events, so caution should be applied when interpreting these values.
According to our definition of immunosuppression (Box 1), 1054 patients were found to have been immunosuppressed before a diagnosis of zoster. Adjusting for prior exposure to immunosuppression had no significant effect on the original hazard ratio of 2.4. The analysis yielded a hazard ratio (risk of subsequent malignancy for the immunosuppressed group taking into account zoster exposure) of 1.23 (95% CI 0.89, 1.68). Hence, there was no evidence that being immunosuppressed had any significant effect on the risk of malignancy.
For the survival analysis, it was only possible to find matches for 573 patients with cancer and a prior diagnosis of zoster. There were 252 deaths in the zoster group and 231 deaths in the no zoster group. Median survival for the zoster group was 1197 days (s.e. 171.8) and median survival for the no zoster group was 1201 days (s.e. 174.8). No significant difference was found; log rank (Mantel-Cox) X2=0.018, df=1, P=0.894.
Discussion
Summary of main findings
This is the largest study of this nature to date, and the first to show a clear association between zoster and a subsequent diagnosis of cancer. Following analysis of the primary care records of 13 248 patients with a diagnosis of zoster, this study shows that the risk of a cancer diagnosis in adults is significantly increased (HR 2.42). The magnitude of the risk varied between cancers, and was highest in younger patients. The median time from zoster to cancer was over 2 years. There were proportionally more cancers in the patients with a history of zoster compared with those without zoster for all age groups and both male and female patients. This was more marked in the first 90 days following diagnosis and in patients over the age of 65 years. A diagnosis of zoster before cancer did not affect survival, although the study was not powered to detect this. Prior immunosuppression was not associated with a change in the risk of cancer; ours being the first study to control for this.
Discussion of findings within context of literature
The studies to which ours are most comparable are those by Sørensen et al (2004), Buntinx et al (2005), and Ho et al (2011). Buntinx et al (2005), in a smaller primary care study from Belgium, demonstrated an increased risk of cancer after zoster, but only in females over the age of 65 years (HR 2.65). Our findings (HR 2.42 for both genders and all age groups) may be a reflection of a much larger sample size (13 248 patients with zoster, compared with 1211). Sørensen et al (2004) had a similar sized sample to ours (10 588 patients, matching 1 : 1.2), but these were patients hospitalised with zoster (and hence less comparable to our findings and those of Buntinx, and less relevant to zoster overall), who were compared with the expected rate (based on age in 10-year age bands, gender, and cancer site) derived from the Danish Cancer Registry. They found an overall relative risk for cancer (including non-melanoma skin cancers) of 1.2 with a cumulative cancer risk of 1.8% in the first year. Similarly to our findings, they found no difference in cancer survival for all cancers but were able to demonstrate a poorer survival for zoster patients with subsequent haematological cancers. Our reported risk is lower than that reported by in the Taiwanese study by Ho et al (2011). Their sample was much smaller and only included patients with herpes zoster ophthalmicus. Our findings differ from those from the large population-based study of Wang et al (2012). This may be explained by study design; ours being a retrospective cohort with matched controls and the Taiwanese study being an unmatched cohort with expected incidence of cancer being the comparator.
It is well established that the incidence of zoster increases with age (Thomas and Hall, 2004; Weinberg, 2007) and for a variety of reasons immunocompetence also declines with age (Arvin, 2005). These facts together with the parallel increase in malignancy with age and an association of zoster with a prior diagnosis of cancer strongly suggest that the immune system is a determinant factor that may link zoster with cancer. Various mechanisms have been suggested for this:
-
There may be a reduction in cell-mediated immunity allowing zoster to manifest itself and a concurrent reduction in immune surveillance for cancer (Buntinx et al, 2005).
-
Zoster may be an early manifestation of an impaired immune system caused by occult cancer (Arvin, 1996; Thomas and Hall, 2004).
-
The zoster virus may provoke an immunological mechanism that weakens immune surveillance for cancer cells allowing tumour escape (Vicari and Trinchieri, 2004; Lin and Karin, 2007), or directly causing cancer (Kuper et al, 2000).
Strengths and limitations
The GPRD is a well-validated primary care data set (Jick et al, 1991) that meets rigorous and regularly applied quality assurance standards. The data are thought to be representative of the UK population both geographically and demographically (Rodríguez and Gutthann, 1998). The size of the database has allowed us to analyse a large number of patients that more than exceeded the number required by the power calculation and is larger than previous studies. We used 5 years of patient data both pre- and post-cancer diagnosis. In the United Kingdom, nearly all zoster is diagnosed and managed within primary care, justifying the use of a primary care data set for this study. Other studies have successfully used the GPRD to identify patients with cancer (Kaye et al, 2002; Jones et al, 2007). We are aware however that the GPRD constitutes data collected in routine practice and can be vulnerable to delays, omissions, and miscoding. Such failings, however, are likely to be similar in both groups in this study. No similar study to date has included immunosuppression as a variable. Our data permitted us to do this, and this is a strength of our work, but this strength was hindered by the fact that we had to develop our own definition of immunosuppression and there are probably a small number of patients on immunosuppressant drugs that fell outside our definition.
A potential weakness of the study is the possibility of an increased diagnostic effort and heightened surveillance both by patients with zoster and by their carers for other disease. This might account for some of the early increased association with a cancer diagnosis (Sørensen et al, 2004), although it seems most unlikely that this would be maintained for several years after a zoster diagnosis. Further weaknesses include our inability to match by year of diagnosis, by practice, or by stage data; hence, the survival comparison between the groups may be open to confounding. Family history of zoster and cancer may also potentially confound our results; it is well established that cancers can be familial and recent evidence suggests that this may also be true for zoster (Hicks et al, 2008). Tobacco consumption, diabetes, and psychological distress have also been linked to immunosuppression and may be confounders. We were unable to adjust for smoking because smoking data in GPRD during the period of the study were incomplete and inaccurate; around one quarter of records have been found to lack data about smoking habits and almost two thirds of former smokers may be misclassified (Lewis and Bresinger, 2004). McDonald et al (2009) have shown a reduced risk of zoster in rheumatoid patients treated with tumour necrosis factor-α antagonists and an increased risk with other immune modulating drugs. Patients with rheumatoid arthritis having a two to five times greater risk of zoster than the general population; thus, this diagnosis might additionally confound our results.
We had to make several assumptions in our methods, and these may have affected the findings. These were our choice of the definition of immunosuppression; our choice of 2 months as the time period between zoster and cancer for the sensitivity analysis; and our choice of 5 years for complete GPRD records before their date of zoster diagnosis or pseudo-diagnosis.
Implications for policy, practice, and research
Previous studies have called for more efforts to detect cancer following a zoster diagnosis (Zaha et al, 1993; Yamamoto et al, 2003); while others have been more cautious (Sørensen et al, 2004; Buntinx et al, 2005). The studies by Ragozzino et al (1982) and Fueyo and Lookingbill (1984) have been cited in reviews of the management of zoster to support the advice that it is not a marker of occult malignancy (Smith and Fenske, 1995; Arvin, 1996; Gnann and Whitley, 2002). Our findings would suggest otherwise and challenge the findings of these previous studies. Some cancers have relatively few early symptoms and signs, and many more initially present with symptoms that are often regarded as self-limiting, or those of benign disease (Hamilton, 2009). Hence, the diagnosis of zoster should raise the index of suspicion for cancer in health-care professionals when presented with symptoms of oncological significance. However, the effect of investigations to try to identify early-stage cancers, and the potential harm caused by the subsequent raising of anxiety are unknown at present. Lastly, the immune system’s response to zoster infection needs further elucidation.
Change history
19 February 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Arvin A (2005) Aging, immunity, and the varicella-zoster virus. N Engl J Med 352: 2266–2267
Arvin AM (1996) Varicella-Zoster virus. Clin Microbiol Rev 9: 361–381
Buntinx F, Wachana R, Bartholomeeusen S, Sweldens K, Geys H (2005) Is herpes zoster a marker for occult or subsequent malignancy? Br J Gen Pract 55: 102–107
Clinical Practice Research Database (2012) www.cprd.com (accessed December 2012)
Fueyo MA, Lookingbill DP (1984) Herpes zoster and occult malignancy. J Am Acad Dermatol 11: 480–482
Gnann JW, Whitley RJ (2002) Herpes zoster. N Engl J Med 347: 340–346
Grambsch P, Therneau T (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81: 515–526
Hamilton W (2009) Five misconceptions in cancer diagnosis. Br J Gen Pract 59: 441–447
Hicks LD, Cook-Norris RH, Mendoza N, Madkan V, Arora A, Tyring SK (2008) Family history as a risk factor for herpes zoster – a case-control Study. Arch Dermatol 144: 603–608
Ho J-D, Xirasagar S, Lin H-C (2011) Increased risk of a cancer diagnosis after herpes zoster ophthalmicus: a nationwide population-based study. Ophthalmology 118: 1076–1081
Hsieh FY, Lavori PW, Cohen HJ, Feussner JR (2003) An overview of variance inflation factors for sample-size calculation. Eval Health Prof 26: 239–257
Jick H, Jick SS, Derby LE (1991) Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ 302: 766–768
Jones R, Latinovic R, Charlton J, Gulliford MC (2007) Alarm symptoms in early diagnosis of cancer in primary care:cohort study using General Practice Research Database. BMJ 334: 1040–1044
Kang J-H, Ho J-D, Chen Y-H, Lin H-C (2009) Increased risk of stroke after a herpes zoster attack a population-based follow-up study. Stroke 40: 3443–3448
Kaye JA, Meier CR, Walker AM, Jick H (2002) Statin use, hyperlipidaemia, and the risk of breast cancer. Br J Cancer 86: 1436–1439
Kuper H, Adami HO, Trichopoulos D (2000) Infections as a major preventable cause of human cancer. J Intern Med 248: 171–183
Lewis JD, Bresinger C (2004) Agreement between GPRD smoking data: survey of general practitioners and a population-based survey. Pharmacoepidemiol Drug Saf 13: 437–441
Lin W, Karin L (2007) A cytokine-mediated link between innate immunity, inflammation and cancer. J Clin Invest 117: 1175–1183
McDonald JR, Zeringue AL, Caplan L, Ranganathan P, Xian H, Burroughs TE, Fraser VJ, Cunningham F, Eisen SA (2009) Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis 48: 1364–1371
Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO (1982) Risk of cancer after herpes zoster: a population-based study. N Engl J Med 307: 393–397
Rodríguez LAG, Gutthann SP (1998) Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol 45: 419–425
Salisbury D, Ramsay M, Noakes K (eds) (2006) Immunosupression. In Immunisation Against Infectious Disease 3rd edn, pp 42–43. TSO: London
Smeeth L, Donnan PT, Cook DG (2006) The use of primary care databases: case-control and case-only designs. Fam Pract 23: 597–604
Smith JB, Fenske NA (1995) Herpes zoster and internal malignancy. South Med J 88: 1089–1092
Sørensen HT, Olsen JH, Jepsen P, Johnsen SP, Schønheyder HC, Mellemkjaer (2004) The risk and prognosis of cancer after hospitalisation for herpes zoster: a population-based follow-up study. Br J Cancer 91: 1275–1279
Thomas SL, Hall AJ (2004) What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis 4: 26–33
Vicari AP, Trinchieri G (2004) Interleukin-10 in viral diseases and cancer: exiting the labyrinth? Immunol Rev 202: 223–236
Wang Y-P, Liu C-J, Hu Y-W, Chen T-J, Lin Y-T, Fung C-P (2012) Risk of cancer among patients with herpes zoster infection: a population-based study. CMAJ 184: E804–E809
Weinberg JM (2007) Herpes zoster: epidemiology, natural history, and common complications. J Am Acad Dermatol 57: S130–S135
Wyburn-Mason R (1955) Malignant change arising in tissues affected by herpes. BMJ 2: 1106–1109
Yamamoto M, Mine H, Akazawa K, MaeharaY SugimachiK (2003) Gastrointestinal cancer and herpes zoster in adults. Hepatogastroenterology 52: 1043–1046
Zaha M, Hayashi I, Odashiro M, Mizogochi H, Fuliwara M, Kato H, Kawamura J (1993) [Herpes zoster and malignancy]. Masui 42: 1343–1346
Acknowledgements
This research was funded by a grant from the British Medical Association (Gunton Award).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Cotton, S., Belcher, J., Rose, P. et al. The risk of a subsequent cancer diagnosis after herpes zoster infection: primary care database study. Br J Cancer 108, 721–726 (2013). https://doi.org/10.1038/bjc.2013.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.13
Keywords
This article is cited by
-
Herpes zoster is not associated with subsequent gastrointestinal cancer: data from over 200,000 outpatients in Germany
Journal of Cancer Research and Clinical Oncology (2023)
-
Increased risk of lymphoid malignancy in patients with herpes zoster: a longitudinal follow-up study using a national cohort
BMC Cancer (2019)
-
Herpes zoster risk after 21 specific cancers: population-based case–control study
British Journal of Cancer (2017)
-
Alpha-Herpesviridae in der Dermatologie
Der Hautarzt (2017)
-
The alpha-herpesviridae in dermatology
Der Hautarzt (2017)