Abstract
Background:
Previous studies suggest that sex steroids influence colorectal cancer (CRC) carcinogenesis. The oestrogen receptor β (ERβ) is the predominantly expressed ER in the colon and loss of ERβ in CRC has been associated with advanced cancer stages.
Methods:
Information on vital status by the end of 2009 was obtained for 1262 CRC patients recruited between 2003 and 2007. The ERβ expression was immunohistochemically measured and associations of ERβ scores with overall survival (OS), disease-specific survival (DSS) and disease-free survival (DFS) were evaluated using Cox proportional hazard models adjusted for prognostic factors, such as tumour stage and second primary tumours.
Results:
Of the 1101 tumour samples with successful measurement, 535 were ERβ negative (48.6%), 381 (34.6%) showed moderate and 185 (16.8%) showed high ERβ expression. Compared with high ERβ expression, lack of ERβ was associated with higher cancer stages as well as greater tumour extent. In multivariate analyses, ERβ negativity was associated with an increased hazard ratio for death (HR=1.61, 95% CI 1.09–2.40, P=0.02), death attributed to CRC (HR=1.54, 95% CI 0.99–2.39, P=0.06) as well as a poorer DFS (DFS HR=1.64, 95% CI 1.23–3.36, P=0.04). The associations were stronger in stage I-III patients (OS HR=2.20, 95% CI 1.28–4.06, P=0.007, DSS HR=2.38, 95% CI 1.20–5.39, P=0.02, respectively).
Conclusions:
Lack of ERβ expression is associated with advanced cancer stages and independently associated with poor survival.
Similar content being viewed by others
Main
The role of oestrogen signalling in colorectal cancer (CRC) remains unclear (Kennelly et al, 2008), although incidence rates are lower in women than in men (Ferlay et al, 2010). Exposure to exogenous hormones through menopausal hormone therapy has consistently been found to be associated with a reduced risk for CRC in postmenopausal women (Hoffmeister et al, 2009; Lin et al, 2012). An abundantly expressed hormone receptor in the normal colonic mucosa is the oestrogen receptor β (ERβ) (Papaxoinis et al, 2010) and ERβ is thought to have a prominent role in the biological mechanisms of sex steroid action on colorectal tissue (Kennelly et al, 2008; Hartman and Gustafsson, 2010). On the other hand, the ERα, which has a major role in breast cancer development (Cuzick et al, 2011), treatment and prognosis (Davies et al, 2011), can be found only at very low levels in normal colorectal tissue (Kennelly et al, 2008). Results of previous studies showed that loss of ERβ expression in CRC is associated with poorer differentiation of tumours and more advanced cancer stages (Konstantinopoulos et al, 2003; Jassam et al, 2005; Elbanna et al, 2012).
Only one previous study investigated the prognostic implications of ERβ expression (Fang et al, 2010). In 423 patients with incident CRC, ERβ-positive tumours were associated with a better overall survival (OS) as well as CRC-specific survival in univariate analyses, but not after adjusting for additional prognostic factors (Fang et al, 2010).
The aim of this study was therefore to evaluate whether ERβ expression is an independent prognostic factor for overall as well as disease-specific survival (DSS) and disease-free survival (DFS) in a large population-based cohort of CRC patients. At the same time, the associations of ERβ expression with tumour and clinical characteristics were assessed.
Materials and methods
Study design and study population
The DACHS study is an ongoing population-based case–control study located in southwest Germany (Lilla et al, 2006; Brenner et al, 2011). Patients with a histologically confirmed first CRC diagnosis as of 1st January 2003 were eligible for recruitment if they were at least 30 years old, physically and mentally able to participate, sufficiently proficient in German and resident in the study region. Written informed consent was given by every study participant. The study was approved by the ethics committee of the University of Heidelberg and the medical boards of Baden–Wuerttemberg and Rhineland–Palatinate. The study population for this investigation comprised cases recruited between 1st January 2003 and 31st December 2007.
In June 2007, formalin-fixed paraffin-embedded (FFPE) surgical specimens of 1564 patients were requested from the pathology departments of the cooperating clinics and transferred to the tissue bank of the National Center for Tumor Diseases in Heidelberg. Samples of 1329 (85%) patients were obtained and 1262 (81%) contained sufficient tumour tissue to be successfully incorporated into tissue microarray (TMA) blocks.
Patients diagnosed with any other cancer (except benign diseases, squamous and basal skin cancer) before their first diagnosis of CRC (N=114), patients who died within 30 days after diagnosis whose death may be related to surgery (N=3) and patients without follow-up information (N=2) were excluded from the current survival analysis (Figure 1). Associations with DFS were evaluated in patients with non-metastatic disease (stage I–III). Therefore, we excluded stage IV patients (N=141) from analyses with DFS as the outcome. Also patients with unknown date of recurrence (N=5) had to be excluded from these analyses (Figure 1).
The study was sufficiently powered (80%) with a type I error probability of 5% to detect a true hazard ratio (HR) of 1.35 for 400 ERβ-negative cases relative to 600 ERβ-positive cases. The power was calculated assuming a recruitment period of 5 years, an additional follow-up period of 3 years and a median survival time of ERβ-positive cases of 7 years.
Data collection and follow-up
The patients gave information during a face-to-face interview conducted by a trained interviewer. The scope of the standardised questionnaire included sociodemographic data, life style and reproductive factors, as well as the family history and medical history of the patients. In addition, discharge letters and pathology reports were collected.
On average 3 years after diagnosis, a questionnaire was sent to the treating physicians of the patients to collect information on CRC therapy, newly diagnosed concomitant diseases and recurrences of CRC. Additional information including again newly diagnosed diseases and recurrences was collected from patients on average five years after diagnosis. After vital status was ascertained, a questionnaire was sent to all patients alive, except those who had denied further contacts. Data on vital status and date of death were obtained from the population registries and the cause of death was verified by death certificates obtained from the health authorities in the Rhein–Neckar–Odenwald region. New diagnoses and cancer recurrences were verified through medical records of the attending physicians.
Study end points
Follow-up time was used as the time variable, and calculated as the time between the date of diagnosis and the date of event or censoring. Death from any cause was the primary end-point. Death attributed to CRC (ICD 10: C18-20) as well as DFS were the secondary end-points. Events of interest with respect to DFS were either recurrent disease or death. Second primary tumours were not counted as events in the DFS analysis. For patients without any event of interest, censoring occurred at the date of last follow-up or 31st December 2009, whichever came first.
Immunohistochemistry
After collecting the requested FFPE samples, they were integrated into TMA blocks, which took place in June 2009. From each surgical specimen four 0.6-mm cores (two cores each from tumour and adjacent non-neoplastic tissue) were punched and integrated into TMA blocks. The 5-μm thick TMA sections were mounted on to superfrost slides. Staining for ERβ was performed in July 2010. The anti-ERβ antibody (primary mouse monoclonal, 14C8, Abcam, Cambridge, UK) was applied at a dilution of 1/50 at room temperature for 30 min. After the incubation with the appropriate biotinylated secondary antibody (Dako antimouse, 1/200 dilution, Dako, Glostrup, Denmark) at room temperature for 15 min and an incubation with the streptavidin avidin–biotin complex kit (Dako), antigen retrieval was performed following endogenous peroxidase blocking. The antibody reactions were revealed using the Dako EnVision+System-HRP. The ERβ expression was visualised with 3,3'-diaminobenzidine (Vector, Peterborough, UK). The lymphocytes in the lamina propria as well as the cores of adjacent non-neoplastic tissue were used as positive control and standard. Sections after the omission of the primary antibody or incubation with the appropriate blocking peptide were used as negative controls. The staining was performed on an autostainer (Dako) based on the avidin–biotin complex method. The sections were counterstained with haematoxylin, dehydrated and coverslipped.
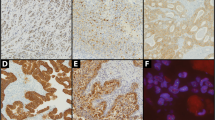
The expression of ERβ in the CRC tissue was independently analysed by two pathologists (CT, WR) blinded to the patient’s outcome. In 96.8% of the cases, results of the scoring were identical. Discrepancies were resolved by an additional joint review of the respective sample. A three-level scoring system (based on Konstantinopoulos et al (2003)) was applied that involved the staining intensity as well as the percentage of positivity in the cancer cell nuclei (Figure 2). Tumours were regarded as negative for ERβ expression, if <10% of the cell nuclei showed positive staining. A moderate expression was defined as weak positive staining of >50% of the cell nuclei or strong positive staining in 10–50% of the nuclei. High expression of ERβ was assigned if >50% of the cell nuclei showed strong positive staining.
Photomicrographs showing the typical staining for the anti-ERβ monoclonal antibody. (A) ERβ expression in adjacent non-neoplastic colonic mucosa; (B) adenocarcinoma negative for ERβ (<10% of nuclei positive); (C) adenocarcinoma showing moderate ERβ expression (10–50% nuclei with strong positive staining or >50% of nuclei with weak positive staining); (D) adenocarcinoma showing high ERβ expression (>50% nuclei with strong positive staining).
Statistical analysis
All analyses were conducted using SAS, version 9.2 (SAS Institute, Cary, NC, USA). Two-sided tests were performed and a P-value of <0.05 was used as significance threshold. Pearson’s χ2 test and the Kruskal–Wallis test were applied to test for differences of clinical parameters and tumour characteristics between patients according to ERβ expression score.
To assess the association of ERβ expression scores with clinical parameters and tumour characteristics, unconditional multinomial logistic regression was carried out. The model was determined using backward selection, retaining variables with a P-value of ⩽0.2. The initial set of variables included tumour extent (T1, T2, T3, T4), nodal status (N0, N1, N2), distant disease (M0, M1), sex, tumour location (colon, rectum), age (in 5-year increments) and former neoadjuvant treatment (yes/no). Samples with missing values in any of the predictor variables or the outcome variable were excluded from the analyses.
Median follow-up time was computed using the reverse Kaplan–Meier method (Schemper and Smith, 1996). To evaluate the association of the ERβ expression scores with OS, DSS, and DFS, regression analyses based on the Cox proportional hazards model were applied. As some patients were interviewed several months after diagnosis, we accounted for possible survival bias by left truncation of the follow-up period. The validity of the model assumptions were assessed by examining plots of Schoenfeld residuals and score processes as well as by including a time-dependent component for each explanatory variable in univariate and multivariate models.
The multivariate models were adjusted for the established prognostic factors such as tumour extent, nodal status, distant disease, age as well as year of diagnosis and stratified by histological grade (well/moderate, poor/undifferentiated). Stratification was performed for all variables showing a time-dependent effect on OS. Again, final models were determined using backward selection, retaining variables with a P-value of ⩽0.2. The final model was additionally stratified for treatment with adjuvant chemotherapy (yes/no) and CRC detection by screening (yes/no) and adjusted for diagnosis of other cancers after CRC diagnosis (yes/no) and BMI (kg m−2, continuous). Kaplan–Meier curves as well as survival curves adjusted (Zhang et al, 2007) according to the final model were used to illustrate the association of ERβ expression scores with OS, DSS, and DFS. Patients with missing values were excluded.
Two sensitivity analyses were performed. First, patients who had received neoadjuvant therapy were excluded. In the second sensitivity analysis, patients with advanced disease (stage IV) were excluded. Owing to the limited number of events when assessing associations with OS and DSS in this latter analysis, Firth’s penalised likelihood approach was applied (Firth, 1993; Heinze and Schemper, 2001).
To evaluate the predictive ability and the validity of the final model, we calculated the concordance probability estimate and R2 and reported the mean values and 95% confidence intervals (CIs) from 1000 bootstrap samples (Nagelkerke, 1991; Gönen and Heller, 2005). We produced receiver-operating characteristic (ROC) curve plots and calculated the area under the ROC curve (AUC) by applying the methods described by Chambless et al (2011).
Results
Measurement of ERβ expression was successful in 1101 of 1262 available surgical samples on TMA (87.2%). Reasons for unsuccessful measurements were an uninformative positive control and loss of cores. Samples with unsuccessful ERβ measurement were more often derived from tumours that were treated with neoadjuvant therapy (15.5% vs 6.0%, P <0.0001) and tumours of T1 and T4 category (T1 9.9% vs 5.9% and T4 17.4% vs 10.9%, P=0.01) than successfully measured samples. Of the 1101 samples with successful measurement, 535 were ERβ negative (48.6%), 381 (34.6%) showed moderate ERβ expression and 185 (16.8%) showed high ERβ expression.
The mean age of the participants was 68.7 years (s.d.: 10.4 years), 57.1% of them were male and 42.9% female. The population characteristics according to ERβ expression score are displayed in Table 1. Tumours negative for ERβ were of higher stage (P=0.003), greater tumour extent (P <0.001) and less often detected by screening (P<0.0001).
The ERβ expression score was still significantly associated with UICC cancer stage and tumour extent after multivariate adjustment (Table 2). Compared with high ERβ expression, negative expression of ERβ was associated with advanced tumour stages (stage II vs I OR=2.45, 95% CI 1.54–3.91, P=0.0002; stage III vs I OR=2.49, 95% CI 1.56–3.98, P=0.0001; stage IV vs I OR=1.79, 95% CI 1.03–3.12, P=0.04) and greater tumour extent (T2 vs T1 OR=2.23, 95% CI 1.10–4.52, P=0.03; T3 vs T1 OR=4.16, 95% CI 2.18–7.92, P<0.0001; T4 vs T1 OR=3.66, 95% CI 1.66–8.08, P=0.001). Similar but weaker associations were found comparing moderate ERβ expression with high ERβ expression.
The median follow-up time of the 1143 patients included in the survival analyses was 4.9 years. During the follow-up period, 346 deaths occurred, including 265 deaths that were attributed to CRC. Further causes of death were other cancers (N=19), cardiovascular disease (N=37) and other causes (N=21). For four participants, the cause of death could not be obtained.
We evaluated the association of the ERβ expression score with OS, DSS and DFS (Table 3). Kaplan–Maier curves as well as survival curves that illustrate the unadjusted and adjusted survival probabilities regarding OS, DSS and DFS are displayed in Figure 3. Compared with having a tumour with high ERβ expression score, the HR associated with having a tumour negative for ERβ expression was 1.61 (95% CI 1.09–2.40, P=0.02) for death from any cause and 1.54 (95% CI 0.99–2.39, P=0.06) for death attributed to CRC. Oestrogen receptor β negativity was also associated with a poorer DFS (HR=1.64, 95% CI 1.23–3.36, P=0.04). The associations for the moderate score of ERβ expression were similar but weaker for OS and DSS (HR for death from any cause: 1.50, 95% CI 0.99–2.27, P=0.06; HR for death attributed to CRC: 1.43, 95% CI 0.89–2.28, P=0.14). However, there was no association of moderate ERβ expression with DFS (HR=1.16, 95% CI 0.71–1.92, P=0.55).
The first sensitivity analysis excluding patients who received neoadjuvant treatment yielded results comparable to those of the main analysis (Table 3). The respective HRs associated with ERβ-negative tumours were similar to those obtained using the whole data set (OS HR=1.62, 95% CI 1.09–2.43, P=0.02; DSS HR=1.56, 95% CI 0.99–2.45, P=0.06 and DFS HR=1.64, 95% CI 1.01–2.67, P=0.05). In the second sensitivity analysis restricted to patients with stage I–III disease, stronger associations than in the main analysis were observed (Table 3). Compared with patients with tumours showing high ERβ expression, patients with ERβ-negative tumours had a significantly associated HR for death of any cause of 2.20 (95% CI 1.28–4.06, P=0.007) and of 2.38 (95% CI 1.20–5.39, P=0.02) for death attributed to CRC.
The multivariate model had a high discriminatory power (CPE=0.73, 95% CI 0.70–0.76). R2 as an additional measure for model validity was 0.47 (95% CI 0.38–0.57), hence the variables in the full model explained 47% of the variance in OS in this study. The inclusion of the ERβ expression score in the model improved the predictive ability of the model slightly (Figure 4A). The AUC value was 0.799 for the model excluding the ERβ expression score and 0.806 for the model including the ERβ expression score. The improvement in predicting OS by including the ERβ score was greater in the subgroup of stage I–III patients, as can be seen by the comparison of the ROC curves (Figures 4A and B). In this patient group, the AUC value was 0.740 for the model without the ERβ expression score and 0.758 for the model including the ERβ expression score.
Discussion
In this prospective patient-cohort study, we found that, in comparison with tumours with high ERβ expression, tumours negative for ERβ were associated with advanced cancer stages. Stage III cancers were 2.5 times more likely to be ERβ-negative in comparison with stage I cancers. Also, tumours of greater extent (T4) were 3.5-fold more likely to show ERβ negativity than T1 tumours. Patients with ERβ-negative tumours had an associated significantly poorer OS with a 61% increased risk of dying compared with patients whose tumours showed high ERβ expression, even after accounting for tumour extent and other prognostic factors.
Lower levels of ERβ mRNA and protein in tumour tissue compared with non-neoplastic tissue have been found consistently in previous studies on CRC, with the percentage of tumours classified as ERβ-negative ranging from 21 to 38% (Foley et al, 2000; Campbell-Thompson et al, 2001; Konstantinopoulos et al, 2003; Jassam et al, 2005; Wong et al, 2005). Results of our study are in line with studies that associated ERβ-negative tumours with advanced tumour stages (Jassam et al, 2005; Elbanna et al, 2012). We did not observe a significantly different expression of ERβ in relation to tumour differentiation as reported by Konstantinopoulos et al (2003). However, sample sizes of previous studies were usually small and ranged from 11 to 91 samples (Foley et al, 2000; Campbell-Thompson et al, 2001; Konstantinopoulos et al, 2003; Jassam et al, 2005; Wong et al, 2005). One relatively large study by Fang et al (2010) including 423 CRC patients also investigated the association of ERβ expression with overall and CRC-specific mortality and reported findings consistent with those from our study. The study population was restricted to patients with stage I-III CRC and the median follow-up time was 86 months. The criteria used to define a tumour as negative for ERβ expression were comparable to ours, although staining of the whole cell rather than the cell nuclei was scored and a different antibody was used. Of the 423 analysed tumour samples, 32.4% were defined as being ERβ-negative and 67.6% as ERβ-positive (including both moderate and high level of ERβ expression). In univariate analyses, Fang et al. found that ERβ-positive tumours were associated with OS (HR=0.58, 95% CI 0.40–0.84, P=0.004) and CRC-specific survival (HR=0.53, 95% CI 0.36–0.79 P=0.001), but the associations were no longer significant after accounting for further prognostic factors. This can be attributed in part to the smaller sample size of the study, yet the magnitude of the reported associations is comparable to that in our study for stage I-III CRC.
We performed a sensitivity analysis excluding patients who had received neoadjuvant therapy, as ERβ expression was less often successfully measured in tumours treated with neoadjuvant therapy. The results did not differ substantially from those obtained based on the whole data set (Table 3). Exclusion of stage IV patients from the analysis yielded larger estimated HRs. Also the improvement of the predictive ability of the model by the ERβ expression score was higher among this subgroup (Figure 4). This is most likely due to a reduction in heterogeneity in the remaining study population with stage I-III patients. The identification of a distant metastasis always leads to a stage IV classification of the tumour, irrespective of its size and differentiation. This heterogeneity in tumour properties of stage IV disease could explain why ERβ negativity was not as strongly associated with stage IV disease as with stage II and stage III disease (Table 2). Furthermore, compared with patients with non-metastatic disease, the survival probability of patients with stage IV disease is very poor overall, and the mostly very short survival times are influenced by additional factors, such as surgical treatment of metastasis (Dahabreh et al, 2011).
We also assessed the association of the ERβ score with DFS in patients with non-metastatic disease. Having a tumour negative for ERβ was associated with a greater risk for disease recurrence or death (Table 3). The association was weaker compared with the association with OS in the same group of stage I–III patients, but similar to that observed with OS in the whole patient group. Hence, the results of the DFS analysis support the associations observed with OS.
Studies on colon cancer cells suggest that ERβ has a role in the regulation of cell proliferation by control of key cell cycle modulators (Martineti et al, 2005; Hartman et al, 2009). In ERβ-knockout mice, cells of the colonic epithelium showed increased proliferation rates, decreased apoptosis as well as less differentiation and cellular adhesion (Wada-Hiraike et al, 2006). Also, an increased incidence of precancerous lesions (aberrant crypt foci) in ERβ-knockout mice has been reported (Saleiro et al, 2010). In a study of ApcMin/+ mice, upregulation of ERβ through a diet containing ERβ-agonists increased the apoptosis rate and normalised the proliferation in the intestinal mucosa (Barone et al, 2010). Taken together, the expression of ERβ seems to be important for the maintenance of the physiologic proliferation of the colonic epithelium, which provides biological plausibility to our results.
Our study had certain strengths and weaknesses. The events of interest were verified by death certificates and medical records, therefore misclassification is unlikely. We were able to account for many factors that are thought to be associated with mortality in CRC patients, including adjuvant therapy and certain co-morbidities. The discriminatory power of the multivariate model was high. However, sufficient data on further potentially important factors such as physical activity (Meyerhardt et al, 2006) and use of NSAIDs (Chan et al, 2009) after diagnosis was not available.
As we did not attempt to repeat the ERβ measurement for samples with unsuccessful measurement, the ERβ score was missing for a relatively large proportion of patients (12.8%). This proportion of failed measurements due to loss of cores and other reasons is not uncommon for immunohistochemistry using TMAs (Jourdan et al, 2003). Our study size was sufficient to compensate the loss of power due to exclusion of samples with missing ERβ score. By using available tissue samples, patient selection might have occurred. However, we did not observe OS to be different for patients with and without TMA samples (HR=1.11, 95% CI 0.74–1.67, P=0.61).
Another common concern with the use of TMAs has been the representativeness of the punched tissue. Using two cores to represent the tumour has been shown to result in sufficient concordance for many different tissue types, including CRC (Jourdan et al, 2003; Giltnane and Rimm, 2004). The antibody 14C8 used in this study recognises most ERβ variants, including the full-length form, and has been shown to be a useful tool for the immunohistochemical assessment of ERβ expression in paraffin-embedded tissue (Skliris et al, 2002; Carder et al, 2005; Speirs et al, 2008). However, splice-variants of ERβ are thought to differ in function from the wild-type ERβ, which is the only form that has shown transcriptional activity (Peng et al, 2003; Leung et al, 2006). The roles of ERβ splice-variants in CRC deserve further research and future studies could potentially gain more detailed insight by using variant-specific antibodies.
To conclude, this is the first study that reports a potential independent prognostic value of ERβ expression in CRC. Results of our study suggest that the loss of ERβ expression is related to CRC progression, and that it is associated with an increased risk of dying, also due to the cancer itself. Additional investigations in prospective patient-cohorts of sufficient size are needed to confirm our findings and to further evaluate the role of ERβ variants in CRC. If the role of ERβ expression in CRC prognosis is confirmed, the prognosis of patients could potentially be improved by therapies aimed at inducing ERβ expression.
Change history
08 August 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Barone M, Tanzi S, Lofano K, Scavo MP, Pricci M, Demarinis L, Papagni S, Guido R, Maiorano E, Ingravallo G, Comelli MC, Francavilla A, Di Leo A (2010) Dietary-induced ERbeta upregulation counteracts intestinal neoplasia development in intact male ApcMin/+ mice. Carcinogenesis 31 (2): 269–274
Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M (2011) Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 154 (1): 22–30
Campbell-Thompson M, Lynch IJ, Bhardwaj B (2001) Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res 61 (2): 632–640
Carder PJ, Murphy CE, Dervan P, Kennedy M, McCann A, Saunders PT, Shaaban AM, Foster CS, Witton CJ, Bartlett JM, Walker RA, Speirs V (2005) A multi-centre investigation towards reaching a consensus on the immunohistochemical detection of ERbeta in archival formalin-fixed paraffin embedded human breast tissue. Breast Cancer Res Treat 92 (3): 287–293
Chambless LE, Cummiskey CP, Cui G (2011) Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat Med 30 (1): 22–38
Chan AT, Ogino S, Fuchs CS (2009) Aspirin use and survival after diagnosis of colorectal cancer. J Am Med Ass 302 (6): 649–658
Cuzick J, DeCensi A, Arun B, Brown PH, Castiglione M, Dunn B, Forbes JF, Glaus A, Howell A, von Minckwitz G, Vogel V, Zwierzina H (2011) Preventive therapy for breast cancer: a consensus statement. Lancet Oncol 12 (5): 496–503
Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA (2011) Systematic review: anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med 154 (1): 37–49
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378 (9793): 771–784
Elbanna HG, Ebrahim MA, Abbas AM, Zalata K, Hashim MA (2012) Potential value of estrogen receptor Beta expression in colorectal carcinoma: interaction with apoptotic index. J Gastrointest Cancer 43 (1): 56–62
Fang YJ, Lu ZH, Wang F, Wu XJ, Li LR, Zhang LY, Pan ZZ, Wan DS (2010) Prognostic impact of ERbeta and MMP7 expression on overall survival in colon cancer. Tumour Biol 31 (6): 651–658
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127 (12): 2893–2917
Firth D (1993) Bias reduction of maximum likelihood estimates. Biometrika 80 (1): 27–38
Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW (2000) Selective loss of estrogen receptor beta in malignant human colon. Cancer Res 60 (2): 245–248
Giltnane JM, Rimm DL (2004) Technology insight: Identification of biomarkers with tissue microarray technology. Nat Clin Pract Oncol 1 (2): 104–111
Gönen M, Heller G (2005) Concordance probability and discriminatory power in proportional hazards regression. Biometrika 92 (4): 965–970
Hartman J, Edvardsson K, Lindberg K, Zhao C, Williams C, Strom A, Gustafsson JA (2009) Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res 69 (15): 6100–6106
Hartman J, Gustafsson JA (2010) Estrogen receptors in colorectal cancer: goalkeepers, strikers, or bystanders? Cancer Prev Res 3 (8): 897–899
Heinze G, Schemper M (2001) A Solution to the problem of monotone likelihood in cox regression. Biometrics 57 (1): 114–119
Hoffmeister M, Raum E, Krtschil A, Chang-Claude J, Brenner H (2009) No evidence for variation in colorectal cancer risk associated with different types of postmenopausal hormone therapy. Clin Pharmacol Ther 86 (4): 416–424
Jassam N, Bell SM, Speirs V, Quirke P (2005) Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes' staging. Oncol Rep 14 (1): 17–21
Jourdan F, Sebbagh N, Comperat E, Mourra N, Flahault A, Olschwang S, Duval A, Hamelin R, Flejou JF (2003) Tissue microarray technology: validation in colorectal carcinoma and analysis of p53, hMLH1, and hMSH2 immunohistochemical expression. Virchows Arch 443 (2): 115–121
Kennelly R, Kavanagh DO, Hogan AM, Winter DC (2008) Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol 9 (4): 385–391
Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG (2003) Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer 39 (9): 1251–1258
Leung YK, Mak P, Hassan S, Ho SM (2006) Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci USA 103 (35): 13162–13167
Lilla C, Verla-Tebit E, Risch A, Jager B, Hoffmeister M, Brenner H, Chang-Claude J (2006) Effect of NAT1 and NAT2 genetic polymorphisms on colorectal cancer risk associated with exposure to tobacco smoke and meat consumption. Cancer Epidemiol Biomarkers Prev 15 (1): 99–107
Lin KJ, Cheung WY, Lai JY, Giovannucci EL (2012) The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer 130 (2): 419–430
Martineti V, Picariello L, Tognarini I, Carbonell SS, Gozzini A, Azzari C, Mavilia C, Tanini A, Falchetti A, Fiorelli G, Tonelli F, Brandi ML (2005) ERbeta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr Relat Cancer 12 (2): 455–469
Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS (2006) Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24 (22): 3527–3534
Nagelkerke NJD (1991) A note on a general definition of the coefficient of determination. Biometrika 78 (3): 691–692
Papaxoinis K, Triantafyllou K, Sasco AJ, Nicolopoulou-Stamati P, Ladas SD (2010) Subsite-specific differences of estrogen receptor beta expression in the normal colonic epithelium: implications for carcinogenesis and colorectal cancer epidemiology. Eur J Gastroenterol Hepatol 22 (5): 614–619
Peng B, Lu B, Leygue E, Murphy LC (2003) Putative functional characteristics of human estrogen receptor-beta isoforms. J Mol Endocrinol 30 (1): 13–29
Saleiro D, Murillo G, Lubahn DB, Kopelovich L, Korach KS, Mehta RG (2010) Enhanced induction of mucin-depleted foci in estrogen receptor {beta} knockout mice. Cancer Prev Res 3 (9): 1198–1204
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17 (4): 343–346
Skliris GP, Parkes AT, Limer JL, Burdall SE, Carder PJ, Speirs V (2002) Evaluation of seven oestrogen receptor beta antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol 197 (2): 155–162
Speirs V, Green CA, Shaaban AM (2008) Oestrogen receptor beta immunohistochemistry: time to get it right? J Clin Pathol 61 (10): 1150–1151
Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson JA (2006) Role of estrogen receptor beta in colonic epithelium. Proc Natl Acad Sci USA 103 (8): 2959–2964
Wong NA, Malcomson RD, Jodrell DI, Groome NP, Harrison DJ, Saunders PT (2005) ERbeta isoform expression in colorectal carcinoma: an in vivo and in vitro study of clinicopathological and molecular correlates. J Pathol 207 (1): 53–60
Zhang X, Loberiza FR, Klein JP, Zhang MJ (2007) A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed 88 (2): 95–101
Acknowledgements
We thank the study participants and the interviewers who collected the data. We also greatly appreciate the help of the hospitals, pathology departments and cooperating institutions in recruiting patients for this study and providing tumour samples. The DACHS study was supported by grants from the German Research Council (Deutsche Forschungsgemeinschaft, grant numbers BR 1704/6-1, BR 1704/6-3, BR 1704/6-4 and CH 390 117/1-1), and the German Federal Ministry of Education and Research (grant numbers 01KH0404 and 01ER0814). This work was also supported by grants from the NGFN+ (Nationales Genomforschungsnetz), grant number 01GS08181 and the Interdisciplinary Research Program of the National Center for Tumor Diseases (NCT), Heidelberg, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Rudolph, A., Toth, C., Hoffmeister, M. et al. Expression of oestrogen receptor β and prognosis of colorectal cancer. Br J Cancer 107, 831–839 (2012). https://doi.org/10.1038/bjc.2012.323
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.323
Keywords
This article is cited by
-
WFDC3 inhibits tumor metastasis by promoting the ERβ-mediated transcriptional repression of TGFBR1 in colorectal cancer
Cell Death & Disease (2023)
-
To Waste or Not to Waste: Questioning Potential Health Risks of Micro- and Nanoplastics with a Focus on Their Ingestion and Potential Carcinogenicity
Exposure and Health (2023)
-
Raloxifene inhibits pancreatic adenocarcinoma growth by interfering with ERβ and IL-6/gp130/STAT3 signaling
Cellular Oncology (2021)
-
In silico studies of the interaction of the colon cancer receptor and RNA aptamer adsorbed on (1 0 1) facet of TiO2 nanoparticle investigated by molecular dynamics simulation
Adsorption (2020)
-
A Molecular Dynamics Study Proposing the Existence of Structural Interaction Between Cancer Cell Receptor and RNA Aptamer
Journal of Inorganic and Organometallic Polymers and Materials (2020)