Abstract
Background:
Gamma-glutamyltransferase (GTT), a known marker for apoptotic balance, seems to promote tumour progression, invasion and drug resistance. Recently, high GGT serum levels were shown to be associated with impaired prognosis in patients with cervical cancer. The aim of this study was to investigate the value of pre-therapeutic serum GGT levels as prognostic parameter in patients with endometrial cancer.
Methods:
Within the present multi-centre trial, clinical–pathological parameters and pre-therapeutic serum GGT levels were evaluated in 874 consecutive patients with endometrial cancer. Patients were stratified in GGT risk groups, and univariate and multivariable survival analyses were performed.
Results:
Mean pre-therapeutic serum GGT level was 30.8 (41.5) U l–1. Elevated and highly elevated serum GGT levels (P=0.03 and P=0.005), tumour stage (P<0.001 and P<0.001), grade (P<0.001 and P=0.02) and age (P<0.001 and P<0.001) were independently associated with progression-free survival in univariate and multivariable survival analyses. Pre-therapeutic GGT was not associated with advanced tumour stage (P=0.6), higher histological grade (P=0.6) or unfavourable histological subtype (P=0.3).
Conclusion:
Pre-therapeutic serum GGT is a novel and independent prognostic parameter for progression-free survival of patients with endometrial cancer. Stratifying patients into prognostic subgroups could be used for patient counselling and individualised treatment planning.
Similar content being viewed by others
Main
The enzyme gamma-glutamyltransferase (GGT) has a substantial role in the metabolism of glutathione (γ-glutamyl-cysteinyl-glycine; GSH). For maintaining adequate levels of intracellular GSH, GGT catalyses the degradation of extracellular GSH, thus providing component amino acids that are then available for further intracellular GSH production. Within the cell GSH functions as a major antioxidant, neutralising reactive oxygen compounds and free radicals (Whitfield, 2001). Gamma-glutamyltransferase expression is found predominantly on the luminal surface of secretory epithelial cells, especially of the hepato-biliary tract, the pancreas and the kidneys. Abnormal GGT expression is found in several human tumours, including breast cancer, ovarian cancer and cervical cancer (Hanigan, 1998). Recently, a possible role of GGT in tumour progression, invasion, drug resistance and prognosis has been suggested (Whitfield, 2001). Underlying mechanisms of GGT on tumour biology are yet still unknown.
In clinical practice, serum GGT is commonly used as a marker for hepato-biliary disease and alcohol intake (Whitfield, 2001). Elevated serum GGT was shown to be associated with all-cause, hepato-biliary, vascular and cancer mortality in both men and women (Kazemi-Shirazi et al, 2007; Ruhl and Everhart, 2009). Moreover, two large prospective epidemiological cohort studies in 79 279 and 92 843 individuals ascertained an association between elevated serum GGT and an increased risk of developing cancer (Strasak et al, 2008a, 2008b). Recently, these results were confirmed in a large Swedish cohort study comprising 545 460 men and women (Van Hemelrijck et al, 2011).
Within the female reproductive tract GGT expression was found in endometrial and endocervical glands. In patients with cervical cancer, elevated serum GGT level was associated with impaired prognosis (Polterauer et al, 2011). We performed the present multi-centre trial to evaluate the association between pre-therapeutic serum GGT and prognosis in a large number of patients with endometrial cancer.
Patients and methods
Patients
A total 874 patients with endometrial cancer were included in the present Austrian multi-centre trial (Department of Gynaecology and Gynaecological Oncology, Comprehensive Cancer Centre, Medical University of Vienna, Vienna, Austria, n=430; Department of Gynaecology and Obstetrics, Medical University Innsbruck, Innsbruck, Austria, n=362; Department of Gynaecology, LKH Klagenfurt, Klagenfurt, Austria, n=82). Clinical and laboratory data were extracted from the respective electronic gynaecologic oncology registries.
Before therapy, a physical examination by a consultant in Internal Medicine and blood tests were performed. The data were documented electronically and in the patients' charts. Patients who presented with pre-existing co-morbidities, which are known to be related to elevated GGT (i.e., alcohol abuse, hepato-biliary tract, pancreatic, and heart disease or alcohol abuse) were excluded from the study. Patients who did not receive standardised treatment because of age or significant co-morbidities were excluded from analysis. Patients with additional, co-existing malignant disease were also excluded from analysis.
Clinical management
Diagnosis of endometrial cancer was established by dilation and curettage. Subsequently, patients were clinically and/or surgically staged according to the International Federation of Gynecology and Obstetrics (FIGO)/American Joint Committee on Cancer classification system of FIGO sixth annual report 2006, using the 1988 FIGO classification (Creasman et al, 2006). Patients were treated and followed according to international guidelines, as described previously (Seebacher et al, 2010).
Pelvic and para-aortic lymphadenectomy was recommended except for FIGO tumour stage Ia and Ib, with histological grade 1 and 2, and endometrioid histology. In patients with high-intermediate-risk or high-risk disease, adjuvant radiotherapy was provided according to standardised treatment protocols (Nag et al, 2000). Adjuvant chemotherapy and/or hormonal therapy were used in selected patients with advanced disease.
If patients did not present for scheduled follow-up visits administrative personnel or nurses contacted them. If any clinically suspicious symptom and/or tumour marker elevation was detected, computed tomography was performed. Recurrent disease was either diagnosed by biopsy or by imaging methods, following standard clinical guidelines.
GGT measurement
Blood samples (serum) were obtained routinely by peripheral venous puncture before therapy during pre-treatment examination. Serum GGT concentrations were analysed with an enzyme kinetic assay (Modular Hitachi 747 and Hitachi 917, Roche Diagnostics, Basel, Switzerland), as described previously (Kazemi-Shirazi et al, 2007).
Statistical analysis
Values are given as means (s.d.). Chi-square test was used to assess the association with obesity and tumour stage. One-way ANOVA was used to assess the association between pre-treatment serum GGT levels and clinical–pathological parameters.
For survival analysis, patients were assigned to previously established GGT risk groups (Franzini et al, 2006; Polterauer et al, 2011), as follows: GGT <17.9 U l–1: group A (normal low); GGT 18.0 to 35.9 U l–1: group B (normal high); GGT 36.0 to 71.9 U l–1: group C (elevated); and GGT >72.0 U l–1: group D (highly elevated). Survival probabilities were calculated by the product limit method of Kaplan and Meier. Differences between groups were tested using the log-rank test. The results were analysed for the endpoint of progression-free survival. Events were defined as cancer-related death or progression at the time of the last follow-up visit. Patients who died of other causes than endometrial cancer or patients alive with no or stable disease were censored with the date of death or last follow-up, respectively. Univariate and multivariable Cox regression models were performed, comprising the GGT risk groups (groups D and C vs groups B and A), FIGO tumour stage (FIGO IV vs FIGO III vs FIGO II vs FIGO I), histological grade (G3 vs G2 vs G1), histological subtype (non-endometrioid carcinoma vs endometrioid adenocarcinoma) and the patients' mean age (>66.7 years vs ⩽66.7 years). Effects of life style factors (obesity, hypertension and diabetes mellitus) and the specific study-centre (Vienna vs Innsbruck vs Klagenfurt) on survival were evaluated by univariate survival analysis. Results of univariate and multivariable survival analyses are given as P-value (hazard ratio and 95% confidence interval). P-values <0.05 were considered statistically significant. We used the statistical software SPSS 16.0 for Mac (SPSS 16.0.1, SPSS Inc., Chicago, IL, USA) for statistical analysis.
Institutional review board
The present trial was approved by the institutional review boards, that is, the ethic committees of the Medical University of Vienna, (Project #248/2009, 21-04-2009), as well as of the Medical University Innsbruck (UN4144).
Results
Patients' characteristics
Patients' baseline characteristics are given in Table 1, clinical–pathological characteristics in Table 2. Mean (s.d.) pre-therapeutic serum GGT level was 30.8 (41.5) U l–1. We used the previously described GGT risk groups (Franzini et al, 2006; Polterauer et al, 2011) for stratification, assigning 356 (40.7%) patients to group A (normal low), 338 (38.7%) patients to group B (normal high), 117 (13.4%) patients to group C (elevated) and 63 (7.2%) patients to group D (highly elevated). We observed a high incidence of obesity, diabetes mellitus and hypertension in our patients' collective (Table 1). Advanced FIGO tumour stages III and IV were less common in obese patients than in patients of normal weight (P=0.009). Patients' characteristics were distributed equally within the three study centres (data not shown).
Association between GGT and clinical–pathological parameters and life style factors
The association between pre-therapeutic serum GGT levels and clinical–pathological parameters is provided in Table 3. We could not show an association between high pre-therapeutic serum GGT and advanced FIGO tumour stage, high histological grade or unfavourable histological subtype. Gamma-glutamyltransferase was not associated with obesity, hypertension, age or diabetes mellitus.
Association between GGT and survival
In univariate analysis, advanced FIGO tumour stage, high histological grade, unfavourable histological subtype, older age and elevated or highly elevated GGT risk groups were associated with poor progression-free survival. In univariate analysis, we did not observe a difference in survival between the three study centres. Obesity, hypertension and diabetes mellitus were not associated with progression-free survival in univariate analysis. Therefore, we did not include these parameters in the multivariable analysis. Elevated or highly elevated GGT risk groups remained independently associated with progression-free survival in multivariable analysis. Results of univariate and multivariable analyses are provided in Tables 4 and 5, respectively.
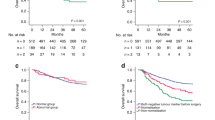
Figure 1 shows Kaplan–Meier curves for pooled GGT risk groups (C+D vs A+B) according to progression-free survival. Patients assigned to the groups of normal low and normal high GGT (A and B) and patients assigned to the groups of elevated and highly elevated GGT (C and D) had 5-year progression-free survival rates of 85.1% and 76.7%, respectively (P=0.03).
Discussion
The present multi-centre trial evaluates the role of pre-therapeutic serum GGT in patients with endometrial cancer. Our data demonstrate an independent association between high pre-therapeutic serum GGT levels and a poor prognosis in patients with endometrial cancer.
We stratified patients into previously described and established prognostic subgroups (Polterauer et al, 2011). Patients of groups A and B (lower GGT serum level) had significantly longer 5-year progression-free survival rates (89.4%) than patients of groups C and D (higher GGT serum level) (76.7%). Our findings seem plausible, as GGT is a known marker of apoptosis and has previously been described to be associated with disease stage and survival in cervical cancer (Polterauer et al, 2011). Our findings are interesting from clinical point of view. Gamma-glutamyltransferase could preoperatively be used for assigning patients to distinctive prognostic subgroups and, if validated in a large independent trial, be incorporated into a nomogram predicting endometrial cancer prognosis.
We assessed the association between GGT, clinical–pathological parameters and life style factors. Interestingly, none of the investigated parameters was associated with GGT. As opposed to previous results on the association between GGT and tumour stage in cervical cancer (Polterauer et al, 2011), we did not observe an association between GGT and disease stage in endometrial cancer. This might reflect differences in tumour genesis and tumour progression between endometrial and cervical cancer. In endometrial cancer, the apparent dissociation between GGT and tumour stage could suggest that GGT might be associated with systemic changes of the disease, for example, inflammation, rather than with the local neoplastic transformation. Additionally, in this study, the number of patients within the respective groups of tumour stage was imbalanced. This is due to the fact that the majority of cases of endometrial cancer present with irregular uterine bleeding as early symptom and are therefore diagnosed in early tumour stage.
Previous studies described that patients with elevated GGT were found to have elevated risk of diabetes (Perry et al, 1998). In addition, GGT was previously shown to be elevated in older patients without cancer (Kazemi-Shirazi et al, 2007). The investigators hypothesise that these findings might be caused by selection bias as only patients with endometrial cancer and no healthy controls were included into our analysis. Nevertheless, we observed a high incidence of symptoms related to metabolic disorders. This is in accordance with previous studies, which showed an increase in endometrial cancer incidence in women with metabolic syndrome compared with healthy women (Friedenrich et al, 2011, Reeves et al, 2011). Interestingly, in our patients collective obese patients showed less cases of advanced tumour stages, which is not supported by previous findings (Reeves et al, 2011).
Experimental studies have explored possible effects of GGT and GSH on tumour cell biology. Traditionally, GGT and GSH have been regarded as essential components of the cell's defence apparatus against oxidative stress. Yet, the distribution and concentration of GGT in several tumours raised the question whether increased GGT expression itself has any active role in neoplastic transformation (Corti et al, 2010). Apparently, under certain conditions, GGT can exert pro-oxidant effects, impairing cellular proliferative/apoptotic balance, thus modulating tumour formation and progression (Corti et al, 2010).
Recently, GSH has been shown to reduce and thereby activate oxidised phosphatase and tensin homolog (PTEN), which acts as a tumour suppressor by inhibiting phosphoinositide 3-kinase-dependent activation of AKT (Kim et al, 2010). Phosphatase and tensin homolog gene mutations are the most frequent genetic lesions in endometrial cancer (83%), causing loss of functional PTEN (Mutter et al, 2000). Most interestingly, Corti et al (2005) showed that GGT promotes S-thiolation of cellular proteins and of proteins of the extracellular environment. The dipeptide cysteinyl-glycin originates from cellular GGT-mediated GSH metabolism and efficiently thiolates proteins. This leads to the formation of cysteinyl-glycine mixed disulphides. Of note, PTEN has been found to undergo glutathionylation (Kim et al, 2010). Thus, we hypothesise that PTEN might as well undergo ‘cysteylglycylation’ mediated by GGT activity. This could possibly lead to a modification or inactivation of PTEN function.
Whether higher GGT possesses a direct biological role in tumour genesis or indirectly reflects increased cellular GGT activity to metabolise extracellular GSH conjugates in accordance to increased demand is yet unclear.
As typical of retrospective studies, our study is limited by biases such as lack of random assignment, patient selection and incomplete data acquisition, although prospectively maintained data bases were extracted. The use of patients from three large cancer centres may reflect a cohort with more aggressive disease, which is the referral pattern for such centres. At the same time, part of our study group performed a prospective cohort study elucidating similar results on the value of GGT as prognostic parameter in patients with endometrial cancer (data not yet published). Our study comprises an even larger number of patients and displays completeness of clinical data. Therefore, despite its retrospective design, our study is of great value.
In endometrial cancer, several biomarkers such as cancer antigen (CA) 125 (Kurihara et al, 1998), CA 15.3 (Lo et al, 1997), C-reactive protein (Schmid et al, 2007) and fibrinogen (Seebacher et al, 2010) have been investigated regarding their impact on prognosis. By elucidating the value of serum GGT as independent prognostic parameter for the survival in patients with endometrial cancer, we provide an additional serum marker, which can be routinely assessed within pre-treatment medical examination.
Change history
23 January 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Corti A, Franzini M, Paolicchi A, Pompella A (2010) Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res 30: 1169–1181
Corti A, Paolicchi A, Franzini M, Dominici S, Casini AF, Pompella A (2005) The S-thiolating activity of membrane gamma-glutamyltransferase: formation of cysteinyl-glycine mixed disulfides with cellular proteins and in the cell microenvironment. Antioxid Redox Signal 7: 911–918
Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY, Pecorelli S (2006) Carcinoma of the corpus uteri. FIGO 6th annual report on the results of treatment in gynecologic cancer. Int J Gynaecol Obstet 95 (suppl 1): 105–143
Franzini M, Corti A, Lorenzini E, Paolicchi A, Pompella A, De Cesare M, Perego P, Gatti L, Leone R, Apostoli P, Zunino F (2006) Modulation of cell growth and cisplatin sensitivity by membrane gamma-glutamyltransferase in melanoma cells. Eur J Cancer 42: 2623–2630
Friedenrich CM, Biel RK, Lau DC, Csizmadi I, Courneya KS, Magliocco AM, Yasui Y, Cook LS (2011) Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev 20 (11): 2384
Hanigan MH (1998) Gamma-glutamyl transpeptidase, a glutathionase: its expression and function in carcinogenesis. Chem Biol Interact 111-112: 333–342
Kazemi-Shirazi L, Endler G, Winkler S, Schickbauer T, Wagner O, Marsik C (2007) Gamma glutamyltransferase and long-term survival: is it just the liver? Clin Chem 53: 940–946
Kim Y, Song YB, Kim TY, Kim I, Han SJ, Ahn Y, Cho SH, Choi CY, Chay KO, Yang SY, Ahn BW, Huh WK, Lee SR (2010) Redox regulation of the tumor suppressor PTEN by glutathione. FEBS Let 584: 3550–3556
Kurihara T, Mizunuma H, Obara M, Andoh K, Ibuki Y, Nishimura T (1998) Determination of a normal level of serum CA 125 in postmenopausal women as a tool for preoperative evaluation and postoperative surveillance of endometrial carcinoma. Gynecol Oncol 69: 192–196
Lo SS, Cheng DK, Ng TY, Wong LC, Ngan HY (1997) Prognostic significance of tumour markers in endometrial cancer. Tumour Biol 18: 241–249
Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, Wenig LP, Eng C (2000) Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 92: 924–930
Nag S, Erickson B, Parikh S, Gupta N, Varia M, Glasgow G (2000) The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the endometrium. Int J Radiat Oncol Biol Phys 48: 779–790
Perry IJ, Wannamethee SG, Shaper AG (1998) Prospective study of serum gamma-glutamyltransferase and risk of NIDDM. Diabetes Care 21: 732–737
Polterauer S, Hofstetter G, Grimm C, Rahhal J, Mailath-Pokorny M, Kohl M, Concin N, Tempfer C, Marth C, Reinthaller A (2011) Relevance of gamma-glutamyltransferase–a marker for apoptotic balance–in predicting tumor stage and prognosis in cervical cancer. Gynecol Oncol 122: 590–594
Reeves KW, Carter GC, Rodabough RJ, Lane D, McNeeley SG, Stefanick ML, Paskett ED (2011) Obesity in relation to endometrial cancer risk and disease characteristics in the Women's Health Initiative. Gynecol Oncol 121: 376–382
Ruhl CE, Everhart JE (2009) Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 136: 477–485. e11
Schmid M, Schneitter A, Hinterberger S, Seeber J, Reinthaller A, Hefler L (2007) Association of elevated C-reactive protein levels with an impaired prognosis in patients with surgically treated endometrial cancer. Obstet Gynecol 110: 1231–1236
Seebacher V, Polterauer S, Grimm C, Husslein H, Leipold H, Hefler-Frischmuth K, Tempfer C, Reinthaller A, Hefler L (2010) The prognostic value of plasma fibrinogen levels in patients with endometrial cancer: a multi-centre trial. Br J Cancer 16: 952–956
Strasak AM, Pfeiffer RM, Klenk J, Hilbe W, Oberaigner W, Gregory M, Concin H, Diem G, Pfeiffer KP, Ruttmann E, Ulmer H (2008a) Prospective study of the association of gamma-glutamyltransferase with cancer incidence in women. Int J Cancer 123: 1902–1906
Strasak AM, Rapp K, Brant LJ, Hilbe W, Gregory M, Oberaigner W, Ruttmann E, Concin H, Diem G, Pfeiffer KP, Ulmer H (2008b) Association of gamma-glutamyltransferase and the risk of cancer incidence in men: a prospective study. Cancer Res 68: 3970–3977
Van Hemelrijck M, Jassem W, Walldius G, Fentiman IS, Hammar N, Lambe M, Garmo H, Jungner I, Holmberg L (2011) Gamma-glutamyltransferase and risk of cancer in a cohort of 545 460 persons–the Swedish AMORIS study. Eur J Cancer 47: 2033–2041
Whitfield JB (2001) Gamma glutamyl transferase. Crit Rev Clin Lab Sci 38: 263–355
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Seebacher, V., Polterauer, S., Grimm, C. et al. Prognostic significance of gamma-glutamyltransferase in patients with endometrial cancer: a multi-centre trial. Br J Cancer 106, 1551–1555 (2012). https://doi.org/10.1038/bjc.2012.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.16
Keywords
This article is cited by
-
Does gamma-glutamyltransferase correlate with liver tumor burden in neuroendocrine tumors?
Endocrine (2023)
-
Prognostic role of gamma-glutamyl transferase in metastatic melanoma patients treated with immune checkpoint inhibitors
Cancer Immunology, Immunotherapy (2021)
-
ɤ-glutamyl hydroxymethyl rhodamine green fluorescence as a prognostic indicator for lung cancer
General Thoracic and Cardiovascular Surgery (2020)
-
Onapristone Extended Release: Safety Evaluation from Phase I–II Studies with an Emphasis on Hepatotoxicity
Drug Safety (2020)
-
Serum gamma-glutamyltransferase and the overall survival of metastatic pancreatic cancer
BMC Cancer (2019)