Abstract
Background:
Cancer cachexia is characterised by skeletal muscle wasting; however, potential for muscle anabolism in patients with advanced cancer is unproven.
Methods:
Quantitative analysis of computed tomography images for loss/gain of muscle in cholangiocarcinoma patients receiving selumetinib (AZD6244; ARRY-142886) in a Phase II study, compared with a separate standard therapy group. Selumetinib is an inhibitor of mitogen-activated protein/extracellular signal–regulated kinase and of interleukin-6 secretion, a putative mediator of muscle wasting.
Results:
Overall, 84.2% of patients gained muscle after initiating selumetinib; mean overall gain of total lumbar muscle cross-sectional area was 13.6 cm2/100 days (∼2.3 kg on a whole-body basis). Cholangiocarcinoma patients who began standard treatment were markedly catabolic, with overall muscle loss of −7.3 cm2/100 days (∼1.2 kg) and by contrast only 16.7% of these patients gained muscle.
Conclusion:
Our findings suggest that selumetinib promotes muscle gain in patients with cholangiocarcinoma. Specific mechanisms and relevance for cachexia therapy remain to be investigated.
Similar content being viewed by others
Main
Cholangiocarcinoma is an uncommon cancer that is associated with a dismal prognosis and significant weight loss and muscle wasting (cancer cachexia; Cooperman et al, 2000; Mosconi et al, 2009). A hallmark of this disease is elevated serum interleukin-6 (IL-6; Goydos et al, 1998) levels, a proinflammatory cytokine that also elicits protein catabolism in skeletal muscle (Bruce and Dyck, 2004). Muscle wasting is a defining feature of cancer cachexia and has major impacts on physical and respiratory function, immunity, chemotherapy response and overall survival (MacDonald et al, 2003; Prado et al, 2008, 2009; Saini et al, 2009; Dodson et al, 2011). Owing to the importance of muscle mass in physiological function and association between muscle loss and outcomes of cancer, alterations in muscle mass as a side effect of anticancer agents is of growing interest. Intracellular signals involved in skeletal muscle anabolism and catabolism have been elucidated. PI3K, AKT and mTOR are central to activating muscle protein synthesis by amino acids (Bodine et al, 2001; Edinger and Thompson, 2002; Saini et al, 2006; Durham et al, 2009). Induction of muscle anabolism by physical activity occurs by pathways involving RAF, MEK and MAPK/ERK kinases (Bodine et al, 2001; Fearon et al, 2011). Cancer therapies directed at these targets would be expected to provoke muscle wasting and this was shown for sorafenib (Antoun et al, 2010). By contrast, some mitogen-activated protein/extracellular signal–regulated kinase kinase (MEK) inhibitors in the development for cancer therapy are anti-inflammatory. Selumetinib (AZD6244, ARRY-142886; AstraZeneca, Manchester, UK), an allosteric inhibitor of MEK1 and MEK2 phosphorylation of ERK (Bekaii-Saab et al, 2011), has tumour suppressive activity in preclinical models (Revill et al, 2006) and has been proven to inhibit IL-6 production (Tai et al, 2007). As proinflammatory cytokines promote muscle protein catabolism (Zaki et al, 2004; Argiles et al, 2009; Murphy and Lynch, 2009), and IL-6 is considered one of the principal catabolic actors in skeletal muscle (Bruce and Dyck, 2004), such agents may mitigate muscle wasting.
In our recent phase II trial of selumetinib (Bekaii-Saab et al, 2011), patients receiving selumetinib experienced an average of 3.9 kg confirmed nonfluid weight gain. Considering the observed weight gain of patients in our phase II study, we investigated muscle and/or fat tissue gain using computed tomography (CT) as described below. The comparator group included patients with advanced cholangiocarcinoma who received standard therapies.
Materials and methods
Studies were approved by Research Ethics Boards of Ohio State University and Alberta Cancer Board.
Selumetinib treatment group
Patients with advanced cholangiocarcinoma participated in a phase II study of selumetinib (100 mg PO b.i.d.; Bekaii-Saab et al, 2011). The formulation was selumetinib-free base in a liquid vehicle Captisol (sulpha-butyl-ethyl B-cyclodextrin). Study inclusion and exclusion criteria have previously been published (Bekaii-Saab et al, 2011).
Standard therapy group
The Cross Cancer Institute is the only cancer centre serving northern Alberta, Canada (population: 1 800 000). A database of all cases (Alberta Cancer Registry) codes primary cancers by site, morphology, clinical and demographic information. For this study, all invasive cholangiocarcinoma cases diagnosed between 1997 and 2007 and included in the Cancer Registry were identified (ICD-10 MO codes: 8140/3, 8141/3, 8160/3, 8162/3, 8180/3) and these were included if they had been evaluated by CT at diagnosis and at least once after starting treatment.
No patients in either group were prescribed anabolic interventions for anorexia-cachexia syndrome (e.g., megesterol acetate, oxandrolone or corticosteroids).
Body composition measurements
Digitally stored CT scans were analysed using Slice-O-Matic software V4.2 (Tomovision, Montreal, Canada). The directly determined measure was cm2 of total skeletal muscle and total adipose tissue at the third lumbar vertebra (L3), a bony landmark previously validated (Mourtzakis et al, 2008) and utilised (Prado et al, 2007, 2008, 2009) in studies of cancer patients. The precision error of measurements is ∼1.5% (Mourtzakis et al, 2008) with a minimum detectable change of approximately 3 cm2.
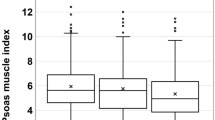
Changes in muscle or adipose tissue are reported as mean cm2 (s.d.) lost or gained over time and also divided into three categories: (A) loss ⩾6.0 cm2, (B) stable ±5.9 cm2 or (C) gain ⩾6.0 cm2 of muscle. These cutoffs are equivalent to loss/gain of ⩾1 kg of skeletal muscle on a whole-body basis (Shen et al, 2004), which are of sufficient magnitude to associate with alterations in muscle strength (Frontera et al, 1988). For adipose tissue, categories were based on the equivalence of 14.7 cm2 total fat at L3 and 1 kg tissue on a whole-body basis (Shen et al, 2004).
Statistics
Data are expressed as mean±s.d. or median/s.e. for continuous variables. Comparisons for categorical variables were conducted using test of proportions, while Student’s t-test was used for continuous variables. Kaplan–Meier curves and log-rank tests were used to compare study groups in relation to survival. Analysis was conducted using SPSS software version 18.0 (SPSS, Chicago, IL, USA). All P-values were two-sided and levels of significance were P<0.05.
Results
Demographics of study participants are described in Table 1. A total of 20 patients from the selumetinib phase II study had images that included the third lumbar vertebra. Patients with cholangiocarcinoma receiving standard treatment (n=30) received the following treatments for either first- or second-line therapy: carboplatin, paclitaxel, etoposide (n=4), gemcitabine with or without capecitabine (n=6), epirubicin, carboplatin, capecitabine (n=4), and radiation (n=7). Nine patients received best supportive care.
The mean interval between scans was 91.5 days for selumetinib-treated patients and 85.5 days for cholangiocarcinoma patients. To account for variation in the exact duration of scan intervals, changes in tissue areas are expressed as: (cm2 lost or gained/number of days between scans) × 100.
Overall, selumetinib-treated cholangiocarcinoma patients gained skeletal muscle, in contrast to those receiving standard therapy, who were markedly catabolic (Table 1, Figure 1); 84.2% of patients gained muscle after initiating selumetinib, compared with 16.7% of patients who were on standard treatment (P<0.001, Figure 1). Selumetinib-treated patients muscle cross-sectional area increased by +13.8 (11.9)cm2/100 days compared with a loss of −7.3 (14.3) cm2/100 days for non-selumetinib-treated patients (P<0.001; Table 1). This translates to approximately +2.3 vs −1.2 kg of skeletal muscle on a whole-body basis, respectively. Tissue gains noted for selumetinib-treated patients were restricted to skeletal muscle (Table 1). Adipose tissue was lost in both groups. There were no observed differences in muscle or adipose tissue changes (gain, stable or loss) between men and women in the standard therapy group vs the selumetinib group (P=0.478 for muscle change and P=0.557 for adipose tissue change).
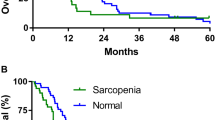
Survival of the two groups is illustrated in Figure 2a. Median time to death was not different between selumetinib vs standard therapy (Table 1, Figure 2A). Because the likelihood of muscle loss increases as death approaches (Lieffers et al, 2009), the selumetinib and standard therapy patients were further compared after stratification by time to death (Figure 2B). Regardless whether patients were started on selumetinib within 150 days of death or earlier, the selumetinib-treated patients showed significant gain of skeletal muscle compared with the standard care group.
Comparison of survival and muscle response to selumetinib therapy or standard treatment in relation to time to death. (A) Survival of patients on selumetinib therapy or on standard treatment. P-value calculated by log-rank test. (B) Mean loss or gain of total lumbar skeletal muscle (cm2) during treatment, overall and stratified by time to death. P-value calculated by T-test.
Discussion
Cholangiocarcinoma is one of the most lethal cancers and is typically associated with cachexia. We show that selumetinib, an agent that holds promising activity in cholangiocarcinoma (Bekaii-Saab et al, 2011), induces rapid and significant skeletal muscle gain. Muscle gain is unanticipated in advanced biliary cancer and was not observed in our comparator group of cholangiocarcinoma patients on standard therapy.
Selumetinib may have direct or indirect action on muscle. Muscle contains both MEK 1 and 2, which are involved in the promotion of myogenic differentiation (Jo et al, 2011). Selumetinib has also been shown to inhibit secretion of cytokines such as IL-6 (Tai et al, 2007), IL-1β and tumour necrosis factor-α, which are implicated in the promotion of cancer cachexia (Zhang et al, 2007, 2008). While the mechanism of action for this anabolic reaction for selumetinib remains unproven, it seems likely that the observed increase in muscle is related to inhibition of cytokine secretion, as inhibition of MEK1/2 would be expected to actually inhibit muscle growth. We previously showed that another tyrosine kinase inhibitor, sorafenib, for example, provokes muscle loss in a randomised, placebo-controlled study (Antoun et al, 2010). In contrast, our current results indicate that the weight gain associated with selumetinib treatment (Bekaii-Saab et al, 2011) is related to increased muscle mass.
Neither patients treated with selumetinib nor those treated standard care group gained adipose tissue. This is consistent with published data demonstrating that muscle and fat are not necessarily gained or lost in concert (Prado et al, 2008). Additionally, a recent international consensus definition of cancer cachexia has characterised cachexia by muscle loss occurring with or without the loss of adipose tissue (Fearon et al, 2011).
A limitation of this work is the lack of a placebo-controlled design. Nonetheless, our results are interesting and indicate a finding consistent across the study that has not been previously described with other biologic or chemotherapeutic agents in various cancers, including cholangiocarcinoma where cachexia is one of the major causes of morbidity and mortality. Our results add to the evidence suggesting that selumetinib is a particularly promising compound in patients with biliary cancer, as previously published (Bekaii-Saab et al, 2011).
These potential benefits for muscle function or other outcomes of selumetinib and potentially of other MEK inhibitors remain to be tested in randomised trials. Future randomised trials with this group of agents should include prospective assessment of inflammatory markers such as IL-6 and other cytokines implicated in cachexia, as well as outcomes that may reveal benefits of skeletal muscle gain. It would be of interest to continue evaluating new targeted cancer therapies for potential actions on muscle. A potential survival benefit of cachexia therapy was raised by the study of Zhou et al (2010), who showed that blocking muscle wasting by antagonism of the action of myostatin can have significant beneficial effects on survival in an animal model of cachexia. This result is currently being tested in a randomised phase II trial in pancreatic cancer (Eli Lilly and Company, 2012).
Change history
23 January 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE (2010) Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol 28: 1054–1060
Argiles JM, Busquets S, Toledo M, Lopez-Soriano FJ (2009) The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care 3: 263–268
Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S (2010) Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 91: 1133S–1137S
Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh JS, O’Neil BH, Balsom S, Balint C, Liersemann R, Vasko VV, Bloomston M, Marsh W, Doyle LA, Ellison G, Grever M, Ringel MD, Villalona-Calero MA (2011) Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 29: 2357–2363
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019
Bruce CR, Dyck DJ (2004) Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab 287: E616–E621
Cooperman AM, Chivati J, Chamberlain RS (2000) Nutritional and metabolic aspects of pancreatic cancer. Curr Opin Clin Nutr Metab Care 3: 17–21
Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, Steiner MS (2011) Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 62: 265–279
Durham WJ, Dillon EL, Sheffield-Moore M (2009) Inflammatory burden and amino acid metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care 12: 72–77
Edinger AL, Thompson CB (2002) Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell 13: 2276–2288
Eli Lilly and Company (2012) A Phase 2 Study of LY2495655 in Participants With Pancreatic Cancer. In: ClinicalTrials.gov [Internet]. National Library of Medicine (US): Bethesda (MD), -[cited 27 Feb 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT01505530 NML identifier: NCT01505530
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, Macdonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489–495
Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ (1988) Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 64: 1038–1044
Goydos JS, Brumfield AM, Frezza E, Booth A, Lotze MT, Carty SE (1998) Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg 227: 398–404
Jo C, Cho SJ, Jo SA (2011) Mitogen-activated protein kinase kinase 1 (MEK1) stabilizes MyoD through direct phosphorylation at tyrosine 156 during myogenic differentiation. J Biol Chem 286: 18903–18913
Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE (2009) A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr 89: 1173–1179
MacDonald N, Easson AM, Mazurak VC, Dunn GP, Baracos VE (2003) Understanding and managing cancer cachexia. J Am Coll Surg 197: 143–161
Mosconi S, Beretta GD, Labianca R, Zampino MG, Gatta G, Heinemann V (2009) Cholangiocarcinoma. Crit Rev Oncol Hematol 69: 259–270
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33: 997–1006
Murphy KT, Lynch GS (2009) Update on emerging drugs for cancer cachexia. Expert Opin Emerg Drugs 14: 619–632
Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, Butts CA, Scarfe AG, Sawyer MB (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13: 3264–3268
Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15: 2920–2926
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9: 629–635
Revill P, Serradell J, Bolos J, Bozzo J (2006) AZD-6244. Drugs Fut 31: 854–858
Saini A, Al-Shanti N, Stewart CE (2006) Waste management—cytokines, growth factors and cachexia. Cytokine Growth Factor Rev 17: 475–486
Saini A, Faulkner S, Al-Shanti N, Stewart C (2009) Powerful signals for weak muscles. Ageing Res Rev 8: 251–267
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97: 2333–2338
Tai YT, Fulciniti M, Hideshima T, Song W, Leiba M, Li XF, Rumizen M, Burger P, Morrison A, Podar K, Chauhan D, Tassone P, Richardson P, Munshi NC, Ghobrial IM, Anderson KC (2007) Targeting MEK induces myeloma-cell cytotoxicity and inhibits osteoclastogenesis. Blood 110: 1656–1663
Zaki MH, Nemeth JA, Trikha M (2004) CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer 111: 592–595
Zhang D, Zheng H, Zhou Y, Tang X, Yu B, Li J (2007) Association of IL-1beta gene polymorphism with cachexia from locally advanced gastric cancer. BMC Cancer7 45
Zhang D, Zhou Y, Wu L, Wang S, Zheng H, Yu B, Li J (2008) Association of IL-6 gene polymorphisms with cachexia susceptibility and survival time of patients with pancreatic cancer. Ann Clin Lab Sci 38: 113–119
Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ (2010) Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142: 531–543
Acknowledgements
We thank Linda Harris for her bibliographic expertise. This study was supported by Grant Support NO1-CM62207, Roche Fellowship in Translational Research from Alberta Health Services (CMMP), Alberta Heritage Foundation for Medical Research Fellowship (CMMP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Prado, C., Bekaii-Saab, T., Doyle, L. et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 106, 1583–1586 (2012). https://doi.org/10.1038/bjc.2012.144
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.144
Keywords
This article is cited by
-
Cancer-associated cachexia — understanding the tumour macroenvironment and microenvironment to improve management
Nature Reviews Clinical Oncology (2023)
-
The prognostic effect of hemoglobin on patients with cancer cachexia: a multicenter retrospective cohort study
Supportive Care in Cancer (2022)
-
Targeting cancer via ribosome biogenesis: the cachexia perspective
Cellular and Molecular Life Sciences (2021)
-
Risk factors and the utility of three different kinds of prediction models for postoperative fatigue after gastrointestinal tumor surgery
Supportive Care in Cancer (2021)
-
Chemotherapy-Induced Sarcopenia
Current Treatment Options in Oncology (2020)