Abstract
Background:
An inverse association between alcoholic beverage intake and risk of renal cell cancer has been suggested in recent studies.
Methods:
We examined the association between alcoholic beverages and renal cell cancer risk in a meta-analysis. We identified relevant studies by searching the database of PubMed, EMBASE, and MEDLINE published through August 2011. We combined the study-specific relative risks (RRs) using a random-effects model.
Results:
A total of 20 case–control studies, 3 cohort studies, and 1 pooled analysis of cohort studies were included in the meta-analysis. We observed that alcoholic beverage intake was associated with a lower risk of renal cell cancer in combined analysis of case–control and cohort studies; for total alcoholic beverage intake, combined RRs (95% confidence intervals) comparing top with bottom categories were 0.76 (0.68–0.85) in case–control studies, and 0.71 (0.63–0.78) in cohort studies (P for difference by study design=0.02). The inverse associations were observed for both men and women and for each specific type alcoholic beverage (beer, wine, and liquor). Also, we found that one drink per day of alcoholic beverage conferred the reduction in renal cell cancer risk, but further drinking above that level did not add benefit.
Conclusion:
The findings from our meta-analysis support the hypothesis that alcoholic beverage intake is inversely associated with a lower risk of renal cell cancer, with moderate consumption conferring the protection and higher consumption conferring no additional benefits.
Similar content being viewed by others
Main
Incidence of renal cell cancer, the major type of kidney cancer, has increased worldwide (Mathew et al, 2002; Chow et al, 2010). Although smoking, obesity, and hypertension are known to be well-established risk factors for renal cell cancer (Chow et al, 2010), dietary modifiable risk factors for renal cell cancer have not been well defined. Alcoholic beverage has been widely consumed and its global consumption has been increasing in the last decades (World Health Organization, 2002). Merits and demerits of alcoholic beverage intake have been long explored because of its benefit for cardiovascular disease (Corrao et al, 2000; Fillmore et al, 2007; Ronksley et al, 2011) or carcinogenic effect (Kan et al, 2011). An International Review panel, sponsored by the World Cancer Research Fund (WCRF) and American Institute for Cancer Research, reported that alcoholic beverage intake increased the risk of cancers of the oral cavity, larynx, pharynx, oesophagus, liver, female breast, and colorectum (World Cancer Research Fund & American Institute for Cancer Research, 2007), but there was a limited evidence about the association between alcoholic beverage intake and kidney cancer.
As the WCRF reported the summary, further studies have examined the association between alcoholic beverage intake and kidney cancer (Greving et al, 2007; Hsu et al, 2007; Lee et al, 2007; Ozasa, 2007; Setiawan et al, 2007; Hu et al, 2008; Pelucchi et al, 2008; Kim et al, 2010; Lew et al, 2011; Allen et al, 2011). A few prospective studies and a pooled analysis of 12 prospective studies found an inverse association for alcoholic beverage intake. However, the associations for men and women were not consistent across studies, partly because of small sample size. Also, there was little evidence whether different types of specific alcoholic beverage including beer, wine, and liquor, confer similar effects.
To elucidate the role of alcoholic beverage intake in renal cell cancer, we investigated the association of total alcoholic beverage and specific alcoholic beverage intake in relation to risk of renal cell cancer in a comprehensive meta-analysis of cohort and case–control studies.
Materials and methods
Search strategy
We identified studies examining the association between alcoholic beverage intake and renal cell cancer by searching the database of PubMed, EMBASE, and MEDLINE published through August 2011. We used the following terms for the PubMed search: ((‘alcohol’ or ‘wine’ or ‘beer’ or ‘liquor’ or ‘ethanol’ or ‘spirit’) and (‘renal cell carcinoma’ or ‘kidney cancer’ or ‘renal cell cancer’ or ‘renal adenocarcinoma’ or ‘kidney adenocarcinoma’)). For the EMBASE and MEDLINE, we used the terms of (alcohol OR wine OR beer OR liquor OR ethanol) AND (carcinoma OR kidney cancer OR cancer OR kidney adenocarcinoma). We included human studies published in English language articles. In addition, we examined the references from the retrieved articles. We confirmed this meta-analysis according to the Meta-analysis of Observational Studies in Epidemiology guidelines (Stroup et al, 2000).
Selection criteria
Two authors (DY Song and S Song) independently assessed the eligibility criteria as follows; (1) case–control or cohort design, published as full-text manuscripts; (2) the exposure of interest was total alcoholic beverage or specific alcoholic beverage intake; (3) the endpoint of interest was renal cell, kidney cancer, renal, or kidney adenocarcinoma; and (4) relative risk (RR) estimates with 95% confidence intervals (CIs) for every category of alcoholic beverage intake or per unit increase in alcoholic beverage intake were reported. For one study (Wynder et al, 1974) that had not examined RRs and 95% CI, we calculated RRs and 95% CI based on the number of cases and controls. When there were multiple publications from the same study population, we only included the study that combined independent studies, examined alcoholic beverage intake as the main interest of exposure, or the study with the largest number of cases or more follow-up years. We did not include two studies (Ozasa, 2007; Kim et al, 2010) in which kidney cancer mortality was endpoint because mortality reflects both incidence and survival.
Data extraction
We extracted from each article the following information: the first author’s last name, publication year, country in which the study was performed, study design, study period, participants’ age and sex, endpoint, exposure assessment, when available, and the number of cases and controls or person–years for each category of alcoholic beverage intake and covariates for adjustment in the analysis. When several estimates were reported, we used the estimates adjusted for the most number of covariates. The authors were contacted for additional information, when necessary. If studies reported the estimate of only specific type of alcoholic beverage (McLaughlin et al, 1984; Asal et al, 1988), we still included those estimates into our meta-analysis of total alcoholic beverage. For one study that reported different types of beer and wine (Greving et al, 2007), we used the estimate of strong beer and strong wine when we analysed the specific type of alcoholic beverages. The quality of each study was assessed by two independent authors (DY Song and S Song) using the Newcastle–Ottawa Scale (Wells et al, 2011) and then averaged. Discrepancies in >1 score between two authors were resolved by consensus.
Statistical analysis
We summarised the estimates across the studies using a random-effects model (DerSimonian and Laird, 1986). We compute the combined RRs and 95% CIs from the estimates reported in each study. We also examined the non-linearity of the relationship using restricted cubic splines (Durrleman and Simon, 1989; Greenland and Longnecker, 1992; Orsini et al, 2012) for studies that provided the number of participants or person–years and two or greater categories of alcoholic beverage intake or ethanol intake. As a result, we included 10 case–control (Wynder et al, 1974; McLaughlin et al, 1984; Asal et al, 1988; Yuan et al, 1998; Mattioli et al, 2002; Parker et al, 2002; Greving et al, 2007; Hsu et al, 2007; Hu et al, 2008; Pelucchi et al, 2008), 3 cohort (Setiawan et al, 2007; Allen et al, 2011; Lew et al, 2011), and 1 pooled analysis studies (Lee et al, 2007) in the spline analysis. To test for non-linearity, we compared the model fit including only the linear term with the model fit including the linear and cubic spline terms using the likelihood ratio test. We used the midpoint between the upper and lower levels in the categories. If the upper level for the highest category was open-ended, we assumed that the level had the same amplitude as the neighbourhood categories.
For the meta-analysis of specific beverages, we rescaled alcoholic beverage into gram (g) of ethanol per day using the conversion factors: 1 drink=15 g, 11.3 g of ethanol for a 4-oz (118 ml) glass of wine, 12.8 g for 12-oz (354 ml) one glass, bottle, or can for beer, and 14.0 g for one measure (45 ml) for liquor. We converted servings per day of alcoholic beverage to 15 g per day of ethanol.
A meta-regression analysis was used to investigate whether the association between alcoholic beverage and risk of renal cell cancer differed by study design (case–control and cohort studies), sex, smoking adjustment (yes, no), or hypertension adjustment (yes, no). Heterogeneity among studies was evaluated by using Q and I2 (Higgins and Thompson, 2002) statistics. The Egger’s regression asymmetry test was used to assess the publication bias (Egger et al, 1997). All analyses were conducted using Stata, version 10.1 (Stata Corp., College Station, TX, USA) and SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). P<0.05 (two-sided) was considered statistically significant.
Results
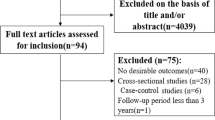
A total of 252 articles were extracted by querying PubMed, EMBASE, and MEDLINE through August 2011 (Figure 1). In all, 174 articles did not examine the association between alcoholic beverage intake and renal cell cancer, 40 were reviews, and 3 were letter, comment, or editorial. Out of 35 articles that examined the association between alcoholic beverage intake and renal cell cancer, 18 were excluded because of data overlap (n=15), the absence of RRs (n=1), assessment of alcoholism as exposure (n=1), and kidney cancer death (n=1). Seven additional studies were identified from the references of the retrieved articles. As a result, 20 case–control studies, 3 cohort studies, and 1 pooled analysis of cohort studies were included in this meta-analysis.
Study characteristics such as country, study design, dates, age, alcohol assessment, unit of alcohol, amount of intake in the beverage for top and bottom categories, outcome/endpoint, number of cases or controls or cohort size, and potential confounders controlled are presented (Tables 1 and 2). A total of 13 819 incident renal cell cancer cases and 1537 incident kidney cancer cases were included in this meta-analysis. Three of twenty-four studies examined incidence of kidney cancer (Wynder et al, 1974; Hsu et al, 2007; Benedetti et al, 2009) and the others examined incidence of renal cell cancer (McLaughlin et al, 1984; Goodman et al, 1986; Asal et al, 1988; Brownson, 1988; Maclure and Willett, 1990; Benhamou et al, 1993; Kreiger et al, 1993; Hiatt et al, 1994; Muscat et al, 1995; Wolk et al, 1996; Boeing et al, 1997; Yuan et al, 1998; Mattioli et al, 2002; Parker et al, 2002; Greving et al, 2007; Lee et al, 2007; Setiawan et al, 2007; Hu et al, 2008; Pelucchi et al, 2008; Allen et al, 2011; Lew et al, 2011). All studies were conducted in the North America and Europe. The alcoholic beverage intake of participations in each study was assessed by using food frequency questionnaire (FFQ), interview, or self-administered questionnaire. The estimates for both men and women were reported in 17 studies. Specific alcoholic beverages were examined in 15 studies. A pooled analysis combined the original data from 12 prospective studies (Lee et al, 2007) conducted in the USA, Finland, Canada, the Netherlands, and Sweden and a multi-centre case–control study (Wolk et al, 1996) combined 4 case–control studies conducted in the Australia, Denmark, Sweden, and USA. Out of 24 studies, all studies adjusted for age, and 14 studies further adjusted for obesity. Smoking status was adjusted for 19 studies. Hypertension status was adjusted for seven studies. Nineteen of twenty-two studies that reported the amount of alcoholic beverage or ethanol as an exposure considered never or non-drinker as the reference and the other 3 studies selected the reference group of 0 or 1 drinks per day (Allen et al, 2011), low to 1 cup per week (Maclure and Willett, 1990), or <1 drink per week (Wolk et al, 1996). The ranges in the top category across studies were from 5 to 120 g per day.
We found a decreased risk of renal cell cancer with alcoholic beverage intake; combined RR (95% CI) comparing top with bottom category was 0.73 (0.67–0.79) for total alcoholic beverage intake (Figure 2). There was no significant heterogeneity across the studies (P for heterogeneity=0.34). A stronger inverse association was observed in cohort studies compared with case–control studies (P for difference=0.02); combined RRs (95% CIs) were 0.76 (0.68–0.85) for case–control studies and 0.71 (0.63–0.78) for cohort studies. When we examined the association by study period (before or after 2000), we found a stronger inverse association for recent studies; comparing top with bottom category, RRs were 0.85 (95% CI, 0.72–0.98; P for heterogeneity=0.32) for earlier studies and 0.70 (95% CI, 0.64–0.76; P for heterogeneity=0.63) for recent studies.
Combined RRs of renal cell cancer for total alcoholic beverage, comparing top with bottom category. McLaughlin et ala examined only beer consumption, Asalb examined only wine consumption. M, F, and C represented male, female, and combined gender, respectively. cP for difference by study design was 0.02.
When we examined specific alcoholic beverages (Table 3), we found that intakes of all three beverage types significantly lowered risk of renal cell cancer. Notably, these inverse associations for each type of alcoholic beverage were observed in both case–control and cohort studies, and the magnitude of the association was similar across three types of alcoholic beverages. There was no evidence of publication bias based on the Egger’s test for beer, wine, or liquor (P>0.19).
When we examined whether the associations differed by gender, adjustment for smoking status, or adjustment for hypertension status, the associations did not vary by these factors (Table 4).
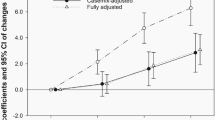
When we examined for non-linearity of the association using the regression cubic spline, we observed a significant non-linearity for overall association between ethanol intake and renal cell cancer. The degree of decline in the risk of renal cell cancer appeared to be attenuated above ∼15 g per day (P for non-linearity<0.001) (Figure 3A). We also found a significant or suggestive non-linearity for case–control and cohort studies (P-values for non-linearity=0.03 for case–control studies and 0.10 for cohort studies; Figures 3B and C).
(A) Combined RR (95% CI) of renal cell cancer and test for the non-linearity of the association using the regression cubic spline. (B) Combined RR (95% CI) of renal cell cancer and test for the non-linearity of the association using the regression cubic spline in case–control studies. (C) Combined RR (95% CI) of renal cell cancer and test for the non-linearity of the association using the regression cubic spline in cohort studies.
Discussion
We observed that alcoholic beverage intake was inversely associated with risk of renal cell cancer in this comprehensive meta-analysis. The inverse association was stronger for cohort studies compared with case–control studies. The inverse associations were consistent across specific alcoholic beverages, suggesting that ethanol per se is most likely the responsible factor. We also found that alcoholic beverage intake lowered risk of renal cell cancer for both men and women. Notably, our spline analysis showed that ∼15 g per day of ethanol intake could pose a decrease in renal cell cancer risk, but additional drinking did not confer further benefit in the prevention of renal cell cancer.
The magnitude of the association was stronger for cohort studies than case–control studies. Recall bias or selection bias in case–control studies may attenuate the association between alcoholic beverage intake and renal cell cancer risk because alcoholic beverage could be overestimated among cases or underestimated among controls. A stronger inverse association for recent studies compared with earlier studies that we observed also could be partly explained by recall or selection bias because all the studies published before 2000 were case–control studies. Also, our meta-analysis of specific type of alcoholic beverage supports the evidence that the benefits associated with alcoholic beverage intake were similar for beer, wine, and liquor.
The possible mechanism by which alcoholic beverage intake reduces the risk of renal cell cancer could be beneficial changes in insulin sensitivity or vasculature. Light to moderate alcohol consumption has been associated with improved insulin sensitivity (Facchini et al, 1994; Davies et al, 2002; Joosten et al, 2008). Increased risk of renal cell cancer among diabetic (Lindblad et al, 1999; Joh et al, 2011) or obese individuals (Chow et al, 2000; Adams et al, 2008) may suggest that factors contributing to the development of insulin resistance are important determinants for renal cell cancer risk. Alcoholic beverage intake may increase high-density lipoprotein cholesterol levels or decrease blood clotting (Booyse et al, 2007) in blood vessels surrounding kidney, a vascular-rich organ.
Our meta-analysis had some limitations. Residual confounding by un-adjustment for confounding factors or inadequate measurement of covariates cannot be ruled out, although the significant inverse association was observed for those adjusted for smoking status and hypertension. Measurement error in assessment of alcoholic beverage intake could attenuate or de-attenuate the association, but the consistent significant inverse associations by different study design, specific types of alcoholic beverage, and gender may not support the possibility that measurement error fully explained our findings in this meta-analysis. The wider CIs observed at heavy alcoholic beverage intake in our spline analysis warrants further studies because of the limited number of renal cell cancer cases for heavy drinking.
In conclusion, we found that alcoholic beverage intake lowered the risk of renal cell cancer with the greatest reduction at the moderate level, but suggesting the evidence that drinking >15 g per day of ethanol does not confer additional benefit for prevention in renal cell cancer risk. Also, reduction of risk was not restricted to any specific type of alcoholic beverages. Moderate alcohol drinking may confer health benefits in the overall survival (Di Castelnuovo et al, 2006), cardiovascular disease (Rimm et al, 1996), and overall health status among elderly (Sun et al, 2011). However, drinking guidelines for men and women from various countries generally limit alcohol drinking to 1–2 standard units of drink (ICAP, 2009) because excessive drinking is associated with increased the risk of birth defects, injury, hypertension, stroke, type 2 diabetes, cancers of oral cavity and pharynx, oesophagus and larynx, stomach, colon and rectum, liver, breast, and ovary (Bagnardi et al, 2001; Reynolds et al, 2003; Baliunas et al, 2009; Taylor et al, 2009; Rehm et al, 2010). Along with the potential for health benefit and risks associated with alcohol consumption, our finding provides the evidence that light to moderate alcohol drinking is enough to reduce renal cell cancer risk without additional benefit above the level of one drink.
Change history
23 January 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Adams KF, Leitzmann MF, Albanes D, Kipnis V, Moore SC, Schatzkin A, Chow WH (2008) Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol 168: 268–277
Allen NE, Balkwill A, Beral V, Green J, Reeves G (2011) Fluid intake and incidence of renal cell carcinoma in UK women. Br J Cancer 104: 1487–1492
Asal NR, Risser DR, Kadamani S, Geyer JR, Lee ET, Cherng N (1988) Risk factors in renal cell carcinoma: I. Methodology, demographics, tobacco, beverage use, and obesity. Cancer Detect Prev 11: 359–377
Bagnardi V, Blangiardo M, La Vecchia C, Corrao G (2001) A meta-analysis of alcohol drinking and cancer risk. Br J Cancer 85: 1700–1705
Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J (2009) Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 32: 2123–2132
Benedetti A, Parent ME, Siemiatycki J (2009) Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: results from a case-control study in Montreal. Cancer Detect Prev 32: 352–362
Benhamou S, Lenfant MH, Ory-Paoletti C, Flamant R (1993) Risk factors for renal-cell carcinoma in a French case-control study. Int J Cancer 55: 32–36
Boeing H, Schlehofer B, Wahrendorf J (1997) Diet, obesity and risk for renal cell carcinoma: results from a case control-study in Germany. Z Ernahrungswiss 36: 3–11
Booyse FM, Pan W, Grenett HE, Parks DA, Darley-Usmar VM, Bradley KM, Tabengwa EM (2007) Mechanism by which alcohol and wine polyphenols affect coronary heart disease risk. Ann Epidemiol 17: S24–S31
Brownson RC (1988) A case-control study of renal cell carcinoma in relation to occupation, smoking, and alcohol consumption. Arch Environ Health 43: 238–241
Chow WH, Dong LM, Devesa SS (2010) Epidemiology and risk factors for kidney cancer. Nat Rev Urol 7: 245–257
Chow WH, Gridley G, Fraumeni JF, Jarvholm B (2000) Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 343: 1305–1311
Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K (2000) Alcohol and coronary heart disease: a meta-analysis. Addiction 95: 1505–1523
Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR (2002) Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA 287: 2559–2562
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled Clin Trials 7: 177–188
Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G (2006) Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 166: 2437–2445
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8: 551–561
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634
Facchini F, Chen YD, Reaven GM (1994) Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care 17: 115–119
Fillmore KM, Stockwell T, Chikritzhs T, Bostrom A, Kerr W (2007) Moderate alcohol use and reduced mortality risk: systematic error in prospective studies and new hypotheses. Ann Epidemiol 17: S16–S23
Goodman MT, Morgenstern H, Wynder EL (1986) A case-control study of factors affecting the development of renal cell cancer. Am J Epidemiol 124: 926–941
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135: 1301–1309
Greving JP, Lee JE, Wolk A, Lukkien C, Lindblad P, Bergstrom A (2007) Alcoholic beverages and risk of renal cell cancer. Br J Cancer 97: 429–433
Hiatt RA, Tolan K, Quesenberry CP (1994) Renal cell carcinoma and thiazide use: a historical, case-control study (California, USA). Cancer Causes Control 5: 319–325
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558
Hsu CC, Chow WH, Boffetta P, Moore L, Zaridze D, Anush M, Janout V, Kollarova H, Bencko V, Navratilova M, Szeszenia-Dabrowska N, Mates D, Brennan P (2007) Dietary risk factors for kidney cancer in eastern and central Europe. Am J Epidemiol 166: 62–70
Hu J, Chen Y, Mao Y, Desmeules M, Mery L (2008) Alcohol drinking and renal cell carcinoma in Canadian men and women. Cancer Detect Prev 32: 7–14
ICAP (2009) Blue Book. International Center on Alcohol Policies. http://www.icap.org/LinkClick.aspx?fileticket=iSBBbvI8INI%3d&tabid=179 (accesed 12 March 2012). Washington, DC.
Joh HK, Willett WC, Cho E (2011) Type 2 diabetes and the risk of renal cell cancer in women. Diabetes Care 34: 1552–1556
Joosten MM, Beulens JW, Kersten S, Hendriks HF (2008) Moderate alcohol consumption increases insulin sensitivity and ADIPOQ expression in postmenopausal women: a randomised, crossover trial. Diabetologia 51: 1375–1381
Kan HP, Huang YQ, Tan YF, Zhou J (2011) Meta-analysis of alcohol consumption and risk of extrahepatic bile system cancer. Hepatol Res 41: 746–753
Kim MK, Ko MJ, Han JT (2010) Alcohol consumption and mortality from all-cause and cancers among 1.34 million Koreans: the results from the Korea national health insurance corporation's health examinee cohort in 2000. Cancer Causes Control 21: 2295–2302
Kreiger N, Marrett LD, Dodds L, Hilditch S, Darlington GA (1993) Risk factors for renal cell carcinoma: results of a population-based case-control study. Cancer Causes Control 4: 101–110
Lee JE, Hunter DJ, Spiegelman D, Adami HO, Albanes D, Bernstein L, van den Brandt PA, Buring JE, Cho E, Folsom AR, Freudenheim JL, Giovannucci E, Graham S, Horn-Ross PL, Leitzmann MF, McCullough ML, Miller AB, Parker AS, Rodriguez C, Rohan TE, Schatzkin A, Schouten LJ, Virtanen M, Willett WC, Wolk A, Zhang SM, Smith-Warner SA (2007) Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst 99: 801–810
Lew JQ, Chow WH, Hollenbeck AR, Schatzkin A, Park Y (2011) Alcohol consumption and risk of renal cell cancer: the NIH-AARP diet and health study. Br J Cancer 104: 537–541
Lindblad P, Chow WH, Chan J, Bergstrom A, Wolk A, Gridley G, McLaughlin JK, Nyren O, Adami HO (1999) The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 42: 107–112
Maclure M, Willett W (1990) A case-control study of diet and risk of renal adenocarcinoma. Epidemiology 1: 430–440
Mathew A, Devesa SS, Fraumeni JF, Chow WH (2002) Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev 11: 171–178
Mattioli S, Truffelli D, Baldasseroni A, Risi A, Marchesini B, Giacomini C, Bacchini P, Violante FS, Buiatti E (2002) Occupational risk factors for renal cell cancer: a case--control study in northern Italy. J Occup Environ Med 44: 1028–1036
McLaughlin JK, Mandel JS, Blot WJ, Schuman LM, Mehl ES, Fraumeni JF (1984) A population-based case-control study of renal cell carcinoma. J Natl Cancer Inst 72: 275–284
Muscat JE, Hoffmann D, Wynder EL (1995) The epidemiology of renal cell carcinoma. A second look. Cancer 75: 2552–2557
Orsini N, Ruifeng L, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and non-linear dose-response relationships: examples, an evaluation of approximations, and software. Am J Epidemiol 175: 66–73
Ozasa K (2007) Alcohol use and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev 8(Suppl:): 81–88
Parker AS, Cerhan JR, Lynch CF, Ershow AG, Cantor KP (2002) Gender, alcohol consumption, and renal cell carcinoma. Am J Epidemiol 155: 455–462
Pelucchi C, Galeone C, Montella M, Polesel J, Crispo A, Talamini R, Negri E, Ramazzotti V, Grimaldi M, Franceschi S, La Vecchia C (2008) Alcohol consumption and renal cell cancer risk in two Italian case-control studies. Ann Oncol 19: 1003–1008
Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, Roerecke M, Room R, Samokhvalov AV, Taylor B (2010) The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction 105: 817–843
Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J (2003) Alcohol consumption and risk of stroke: a meta-analysis. JAMA 289: 579–588
Rimm EB, Klatsky A, Grobbee D, Stampfer MJ (1996) Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits. BMJ 312: 731–736
Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA (2011) Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 342: d671
Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE (2007) Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol 166: 932–940
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012
Sun Q, Townsend MK, Okereke OI, Rimm EB, Hu FB, Stampfer MJ, Grodstein F (2011) Alcohol consumption at midlife and successful ageing in women: a prospective cohort analysis in the nurses' health study. PLoS Med 8: e1001090
Taylor B, Irving HM, Baliunas D, Roerecke M, Patra J, Mohapatra S, Rehm J (2009) Alcohol and hypertension: gender differences in dose-response relationships determined through systematic review and meta-analysis. Addiction 104: 1981–1990
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2011) The Newcastle–Ottawa Scle (NOS) for assessing the quality of nonrandomised sutdies in meta-analysis, Dept of Epidemiology and Community Medicine, University of Ottawa: Ottawa, Canada, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 10 February 2011).
Wolk A, Gridley G, Niwa S, Lindblad P, McCredie M, Mellemgaard A, Mandel JS, Wahrendorf J, McLaughlin JK, Adami HO (1996) International renal cell cancer study. VII. Role of diet. Int J Cancer 65: 67–73
World Cancer Research Fund, American Institute for Cancer Research (2007) Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. American Institute for Cancer Research: Washington, DC
World Health Organization (2002) The World Health Report 2002 - Reducing Risks, Promoting Healthy Life. World Health Organization: Geneva, Switzerland
Wynder EL, Mabuchi K, Whitmore WF (1974) Epidemiology of adenocarcinoma of the kidney. J Natl Cancer Inst 53: 1619–1634
Yuan JM, Gago-Dominguez M, Castelao JE, Hankin JH, Ross RK, Yu MC (1998) Cruciferous vegetables in relation to renal cell carcinoma. Int J Cancer 77: 211–216
Acknowledgements
This work was supported by the Sookmyung Women’s University Research Grant (2010) and the Brain Korea 21 (BK 21) Project from the Ministry of Education and Human Resources Development, Republic of Korea. We thank Dr Setiawan for the data provision.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Song, D., Song, S., Song, Y. et al. Alcohol intake and renal cell cancer risk: a meta-analysis. Br J Cancer 106, 1881–1890 (2012). https://doi.org/10.1038/bjc.2012.136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.136
Keywords
This article is cited by
-
The association of healthy lifestyle index score and the risk of renal cell cancer in the Netherlands cohort study
BMC Cancer (2023)
-
Effect of smoking, hypertension and lifestyle factors on kidney cancer — perspectives for prevention and screening programmes
Nature Reviews Urology (2023)
-
Genomic and epigenomic integrative subtypes of renal cell carcinoma in a Japanese cohort
Nature Communications (2023)
-
The role of diet in renal cell carcinoma incidence: an umbrella review of meta-analyses of observational studies
BMC Medicine (2022)
-
Coffee consumption and risk of renal cancer: a meta-analysis of cohort evidence
Cancer Causes & Control (2022)