Abstract
Background:
In advanced renal cell carcinoma (RCC), sunitinib and sorafenib tyrosine kinase inhibitors (TKI) are associated with several clinical side effects, with no definitive established data concerning their clinical impact.
Methods:
From June 2006 to June 2008, main clinical TKI-induced toxicities, including digestive, cardiac, dermatologic and asthenia were retrospectively collected using the NCI-CTC version 3.0 in patients treated with TKI for an RCC.
Results:
The median overall survival was significantly improved in patients with grade 3–4 clinical toxicities (36 vs 12 months, P=0.009). In multivariate analysis, the Memorial Sloan-Kettering Cancer Center risk groups (good vs intermediate or poor) and clinical toxicities (grade 3–4 vs 1–2) were identified as independent prognostic factors of better survival (P=0.002 and P=0.02, respectively). The Charlson comorbidity index score (>7 vs <7) was identified as independent predictive factor of severe clinical TKI-induced toxicities (P=0.02).
Conclusion:
In this unselected patients of RCC, clinical TKI-related severe toxicities were more frequent in patients with comorbidities and were associated with better survival.
Similar content being viewed by others
Main
Sunitinib (SU011248) and sorafenib (BAY 43-9006) are two oral multitargeted tyrosine kinase inhibitors (TKIs) available since late 2006 and early 2007, and both have been subsequently used as first-line agents in selected patients with advanced renal cell carcinoma (RCC; Escudier et al, 2007, 2010; Motzer et al, 2007; Powles et al, 2011). In randomised trials, the more frequent clinical adverse events reported with sunitinib and sorafenib were, respectively, fatigue in 51% and 37%, gastrointestinal disorders including diarrhoea in 53% and 48%, and nausea in 44% and 23%, hypertension in 24% and 17%, skin toxicities with rash or desquamation in 19% and 40%, and hand–foot syndrome in 20% and 30% of cases. Some grade 3–4 were also observed with respectively diarrhoea in 5% and 2%, nausea in approximately 3%, hypertension in 8% and 4%, rash or desquamation in less than 1%, hand–foot syndrome in 6% and 8%, and fatigue in 7% and 5% (Escudier et al, 2007; Motzer et al, 2007). Until now, analysis of TKI-induced toxicities has been mainly descriptive, using a global evaluation and counting of adverse events occurring in patients selected for randomised studies. On the basis of this approach, the impact of TKI related-toxicities on outcome has not yet been definitively established. Moreover, active monitoring for adverse reactions, careful management of related toxicities and dose adaptation during TKI exposure are recommended, but are not yet modulated according to patient characteristics (Escudier et al, 2010; Schmidinger and Bellmunt, 2010; Bellmunt et al, 2011). We retrospectively evaluated the predictive factors and the impact on the outcome of this grade 3–4 clinical TKI toxicities in an unselected population of patients treated for RCC.
Materials and methods
From June 2006 to June 2008, all consecutive patients with RCC treated with sunitinib or sorafenib were included and classified according to the Memorial Sloan-Kettering Cancer Center (MSKCC) risk score (Motzer et al, 1999). For each patient, we collected all clinical characteristics, as well as toxicities according to NCI-CTC version 3. Comorbidities present at TKI initiation such as hypertension, diabetes, dyslipidemia, renal insufficiency, alcohol consumption and previous history of cancer were also analysed, and the Charlson comorbidity index was calculated as previously described (Charlson et al, 1987). One cycle of sunitinib consisted of 4 consecutive weeks followed by 2 weeks break (dose of 50 mg per day), whereas one cycle of sorafenib consisted of 4 consecutive weeks without discontinuation (dose of 400 mg twice daily). Patient follow-up was routinely performed at day 1, 14 and 28 of the first treatment cycle, and at least, monthly during TKI exposure. Predictive factors of the main clinical toxicities regarding digestive, cardiac, dermatologic and asthenia adverse events occurring during TKI sequences and prognostic factors of overall survival (OS) were respectively analysed, using a logistic regression and a Cox model.
Results
We analysed 53 TKI sequences from 38 patients; 14 received sunitinib, 9 received sorafenib and 15 received both TKI (Table 1). The mean time of exposure was 268±298 days, with 313±317 days for sunitinib and 213±271 days for sorafenib (NS). All toxicities grades were observed in 88.7% of all sequences with digestive in 73.6%, cardiac in 64.1%, dermatologic in 52.3% and asthenia in 52.3%. Grade 3–4 toxicities were observed in 51%, and the most frequent were cardiac in 31.2% and dermatologic in 8.6%. Grade 3–4 cardiac toxicities were more frequent during sunitinib sequences (45% vs 12.5%, P=0.02), whereas grade 3–4 dermatologic effects and percentage of dose reduction were more often observed during sorafenib (33.3% vs 7%, P=0.03 and 40.6% vs 17.2%, P=0.02). In the multivariate analysis including gender, MSKCC risk groups, type of TKI, tumour grade and the Charlson comorbidity index score (>7 vs <7), the comorbidity index score was identified as an independent predictive factor of TKI-induced toxicities (P=0.02, HR: 4.48; IC 95: 1.18–16.9; Table 1).
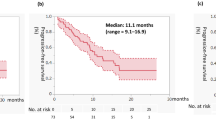
According to the MSKCC score, the 2-year survival rate was 78% in patients with low and intermediate vs 36% in poor risk (P=0.05). The median OS was 36 months in patients with grade 3–4 clinical toxicities as compared with 12 months in patients without toxicities (P=0.009; Figure 1). Moreover, in patients with an age-adjusted Charlson score >7, the median OS was 12 months vs 36 months in patients Charlson score <7 (P=0.009). In patients with WHO performance status at 2, 1 and 0, the median OS was 6 months vs 9 months vs 39 months, respectively (P=0.0005). In the multivariate analysis, the MSKCC risk groups (good vs intermediate or poor) and severe clinical toxicities (grade 3–4 vs grade 1–2) were identified as independent prognostic factors of survival (P=0.002; HR: 0.10; IC 95: 0.02–0.43 and P=0.02; HR: 5.55; IC 95: 1.23–24.9, respectively; Table 2).
Discussion
Considering the sample size and the retrospective nature of the series, our results suggest that grade 3–4 clinical TKI-related toxicities namely digestive, cardiac, dermatologic and asthenia were associated with a significant improvement of OS. In a series of 40 patients with RCC, Rixe et al (2007) have reported that toxicities limited to grade 3 hypertension was associated with response and outcome in patients treated with sunitinib. More recently, Rini et al (2011) reported in a retrospective pooled analysis from four studies of patients with RCC that sunitinib-associated hypertension was associated with improved clinical outcomes. Interestingly, survival rates were close to those observed in our work with a median OS at 30.9 months in patients who experienced hypertension vs 7.2 months in patients who did not. Some similar observations were reported in other malignancies, suggesting a potential prognostic impact of the main target therapies-related side effects. In advanced intestinal stromal digestive tumours, George et al (2011) reported that hypertension level was a predictive factor of response in patients treated with imatinib. In metastatic colorectal cancer patients treated with anti-EGFR monoclonal antibodies, it has been reported that skin rash may be a prognostic factor, and a study is currently ongoing to evaluate the anti-EGFR dose escalation according to skin toxicity (Van Cutsem et al, 2009). In contrast to monoclonal antibodies, TKIs inhibit multiple tyrosine kinase and were, in fact, associated with several non-VEGF-related biological and clinical side effects such as those investigated in our study. Although the exact mechanisms of these non-VEGF-related side effects are currently undetermined, several factors have been proposed such as the binding affinities for tyrosine kinase receptors, the cellular level of these receptors, and also previous treatment and pre-existing comorbidities (Schmidinger and Bellmunt, 2010). Combination of these factors could partially explain the different toxicity between TKI that we observed with more frequent grade 3–4 cardiac toxicities during sunitinib and more dermatological side effects during sorafenib. We also found that patient comorbidities may be associated with grade 3–4 clinical TKI-induced toxicities and was also correlated with OS. In a study on unselected patients treated with sunitinib, van der Veldt et al (2008) reported that occurrence of overall grade 3–4 toxicities was significantly associated with age, body surface and gender, but the Charlson comorbidity index was not used, and the impact on survival was also not reported. In contrast, we added the Charlson comorbidity index to other common baseline parameters and we found that it was significantly associated with clinical grade 3–4 TKI toxicities. Until now, the most widely used clinical score is the Charlson comorbidity index (Charlson et al, 1987). This score was constructed using a study of 559 patients and its ability to predict the 1-year mortality was secondary validated on a cohort of women with breast cancer. The non-adjusted score encompassing 19 medical conditions weighted 1–6, with total scores ranging from 0 to 37. Age was also identified as a prognostic factor in the validation set with one point added to the score for each decade of life over the age of 50 (Charlson et al, 1987). Whatever the malignancy, randomised trials do not strictly reflect patient characteristics from cohort routinely treated in cancer units. Indeed, patients included in these studies often presented a good general health status, whereas patients with several comorbid conditions are preferentially referred to other therapeutics. As a result, in previous randomised trials using sunitinib and sorafenib in RCC, patients were analysed according to common baseline characteristics, but the evaluation of comorbid conditions by specific index, such as Charslon score, and the impact on tolerance and outcome have not been performed (Escudier et al, 2007; Motzer et al, 2007). These findings suggested that clinical TKI related-side effects may be in relation with patient conditions and may be also a marker of drug efficacy. Therefore, early and intensive monitoring during treatment exposure remains a major concern for a careful toxicity management, as well as dose adaptation. As regards the potential prognostic impact of TKI-induced side effects, evaluation of specific supportive measures, particularly in patients with high risk of toxicities, may be of interest, to ensure an optimal drug exposure in the field of sequential use of biological agents in RCC.
References
Bellmunt J, Eisen T, Fishman M, Quinn D (2011) Experience with sorafenib and adverse event management. Crit Rev Oncol Hematol 78: 24–32
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383
Escudier B, Albiges L, Blesius A, Loriot Y, Massard C, Fizazi K (2010) How to select targeted therapy in renal cell cancer. Ann Oncol 21 (Suppl 7): vii59–vii62
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, TARGET Study Group (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: 125–134
George S, Lechner T, Li S, Cohen DP, Demetri GD (2011) Hypertension (HTN) as a potential biomarker of efficacy in patients (pts) with gastrointestinal stromal tumor (GIST) treated with sunitinib (SU). Gastrointestinal Cancers Symposium. J Clin Oncol 29 (Suppl 4): abstract 38
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124
Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17: 2530–2540
Powles T, Chowdhury S, Jones R, Mantle M, Nathan P, Bex A, Lim L, Hutson T (2011) Sunitinib and other targeted therapies for renal cell carcinoma. Br J Cancer 104: 741–745
Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, Figlin RA, Baum MS, Motzer RJ (2011) Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 103: 763–773
Rixe O, Billemont B, Izzedine H (2007) Hypertension as a predictive factor of Sunitinib activity. Ann Oncol 18: 1117–1125
Schmidinger M, Bellmunt J (2010) Plethora of agents, plethora of targets, plethora of side effects in metastatic renal cell carcinoma. Cancer Treat Rev 36: 416–424
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360: 1408–1417
van der Veldt AA, Boven E, Helgason HH, van Wouwe M, Berkhof J, de Gast G, Mallo H, Tillier CN, van den Eertwegh AJ, Haanen JB (2008) Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br J Cancer 99: 259–265
Acknowledgements
The authors are grateful to Richard Medeiros, Rouen University Hospital Medical Editor, for his valuable editorial assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Di Fiore, F., Rigal, O., Ménager, C. et al. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer 105, 1811–1813 (2011). https://doi.org/10.1038/bjc.2011.507
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2011.507
Keywords
This article is cited by
-
TKIs beyond immunotherapy predict improved survival in advanced HCC
Journal of Cancer Research and Clinical Oncology (2023)
-
Exposure–response analyses of cabozantinib in patients with metastatic renal cell cancer
BMC Cancer (2022)
-
Predictive Markers of First Line Pazopanib Treatment in Kidney Cancer
Pathology & Oncology Research (2020)
-
Validation of a Simple Scoring System to Predict Sorafenib Effectiveness in Patients with Hepatocellular Carcinoma
Targeted Oncology (2017)
-
Identification of clinical biomarkers for patients with advanced hepatocellular carcinoma receiving sorafenib
Clinical and Translational Oncology (2017)