Abstract
Background:
We aimed to evaluate the clinical relevance of p53 and p73 isoforms that modulate the function of p53.
Methods:
This prospective multicentre study included 154 patients with stage III and IV serous ovarian cancer. A functional yeast-based assay and subsequent sequencing were performed to analyse the p53 mutational status. Expression of p53 and p73 isoforms was determined using RT–qPCR.
Results:
Δ133p53 expression constituted an independent prognostic marker for recurrence-free (hazard ratio=0.571, P=0.016, 95% CI: 0.362–0.899) and overall survival (hazard ratio=0.365, P=0.004, 95% CI: 0.182–0.731) in patients with p53 mutant ovarian cancer (n=121). High Δ40p53 expression was associated with favourable tumour grading (P=0.037) and improved recurrence-free survival (33.4 vs 19.6 months, P=0.029), but not overall survival (43.1 vs 33.6 months, P=0.139), in patients with p53 wild-type cancer (n=33). Neither the p53 mutational status nor p73 isoform expression possessed prognostic significance in the examined ovarian cancer cases.
Conclusion:
Δ133p53 expression was associated with prognosis in the vast majority of ovarian cancer cases, that is, patients with p53 mutant advanced serous carcinomas. Thus, our findings underline the importance of considering the complex p53 regulatory network.
Similar content being viewed by others
Main
Recently, the N-terminally truncated p53 isoforms Δ40p53 and Δ133p53 were added to the complex network that modulates the function of the tumour suppressor p53. Δ40p53 is generated by alternative splicing of intron 2 or altered initiation of translation in exon 4 (Courtois et al, 2002; Yin et al, 2002; Ghosh et al, 2004), whereas Δ133p53 is derived from an alternative promoter located in intron 4 (Bourdon et al, 2005). Δ40p53 lacks the first 40 amino acids, but retains the second transactivation domain (Murray-Zmijewski et al, 2006). By contrast, Δ133p53 is devoid of both transactivation domains and part of the DNA-binding domain (Figure 1). Both p53 isoforms contain the C-terminal tetramerisation domain, allowing their incorporation into p53 tetrameres. The exact function of Δ40p53 and Δ133p53 has not yet been fully characterised. The present consensus is that their ratio to FLp53 determines functional outcome (Jänicke et al, 2009). Low Δ40p53 and Δ133p53 levels have been reported to act as potent dominant-negative inhibitors of FLp53, suppressing transcription of genes under the control of a p53-binding element as well as p53-induced apoptosis (Yin et al, 2002; Bourdon et al, 2005; Graupner et al, 2009). Contrarily, high Δ40p53 and Δ133p53 levels have resulted in enhanced transcription of FLp53 target genes (Chen et al, 2005; Ohki et al, 2007; Powell et al, 2008). Studies analysing the expression patterns of N-terminally truncated p53 isoforms in distinct types of carcinomas are rare and have not shown a clinical relevance of p53 isoforms (Boldrup et al, 2007; Avery-Kiejda et al, 2008; Ebrahimi et al, 2008; Chen et al, 2009; Song et al, 2009).
Gene architecture of the N-terminus of the p53 gene, indicating the mode of generation for the various p53 isoforms as well as their resulting protein structure (TA, transactivation domain (blue); PR, proline-rich domain (red); DBD, DNA-binding domain (yellow); untranscribed regions (white); introns (grey)).
Similarly, the p53 family member p73 gives rise to multiple isoforms. Alternative splicing of the P1 promoter transcript generates both the full-length TAp73 as well as ΔN7prime;p73, whereas an alternative P2 promoter in intron 3 produces ΔNp73. Importantly, ΔN′p73 and ΔNp73 transcripts encode the same protein product lacking the transactivation domain. This N-terminally truncated p73 protein ΔNp73 acts as a powerful dominant-negative inhibitor of both wild-type p53 and TAp73, either by direct competition for DNA binding sites or by the formation of heterocomplexes (Bailey et al, 2011). ΔNp73 has been an independent prognostic marker in distinct types of carcinomas (Uramoto et al, 2004; Müller et al, 2005; Liu et al, 2006; Vilgelm et al, 2010). In ovarian cancer, we previously reported that expression of N-terminally truncated p73 isoforms has a role in response to platinum-based chemotherapy and constitutes an independent prognostic marker in patients with p53 mutant ovarian cancer (Concin et al, 2005).
Most existing studies in ovarian cancer comprise patient groups heterogeneous for well-known prognostic factors such as FIGO stage and histological subtypes. While 5-year survival rates can be as high as 80–95% among patients with early-stage disease (stage I or II), patients with advanced carcinomas (stage III or IV) have survival rates of 10–30% (Cannistra, 2004). Recently, the histological subtype was reported to be an independent prognostic marker in patients with stage III ovarian cancer. Mucinous and clear cell carcinomas possessed an impaired prognosis as compared with serous ovarian carcinomas, whereas endometrioid carcinomas showed favourable prognosis (Winter et al, 2007). In addition, histological subtypes display different biomarker expression profiles, thus further supporting the hypothesis that histological subtypes represent different disease entities. For instance, serous carcinomas show WT1, Mesothelin, oestrogen receptor and CA125 expression in >75%, while the mucinous subtype displays frequent expression of Matriptase, and endometrioid carcinomas express high rates of estrogen and progesterone receptor and CA125 (Köbel et al, 2008). Heterogeneous patient groups might substantially confound results of biomarker studies and constitute an important reason for the difficulty experienced in confirming potential prognostic markers in subsequent studies.
Thus, a homogeneous cohort of patients with primary advanced serous ovarian cancer was prospectively recruited within the multicentre study OVCAD (OVarian CAncer Diagnosis of a silent killer). We aimed to evaluate the clinical relevance of p53 (Δ133p53, Δ40p53, and FLp53) and p73 isoforms (TAp73 and ΔTAp73) that modulate the function of p53 in patients with advanced serous ovarian cancer.
Patients and methods
Patients and tissue samples
Between August 2005 and December 2008, 154 consecutive patients diagnosed with primary advanced serous ovarian cancer at the Departments of Gynecology and Obstetrics in Berlin (n=55), Leuven (n=50), Hamburg (n=26), Vienna (n=19), and Innsbruck (n=4) were enrolled in the OVCAD project, a Sixth Framework Program Project of the European Union (http://www.ovcad.eu). The Ethics Committees of the respective centres approved the study protocol. All patients signed written informed consent before enrollment.
RNA isolation and real-time RT–PCR
Tissue samples were obtained at the time of diagnosis and immediately stored in liquid nitrogen. RNA isolation was done by 1600 nucleic acid prepstation (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions including a DNase digestion step. Eluted RNA was precipitated with ethanol and resuspended in RNase-free water. Reverse transcription was performed as described previously (Reimer et al, 2007).
Primer pairs and probes for p53 (Δ40p53, Δ133p53, and FLp53) and p73 isoforms (TAp73 and ΔTAp73, which recognises both ΔNp73 and ΔN’p73 transcripts) as well as the internal control TBP (TATA box-binding protein) were designed using Primer Express software (Applied Biosystems; Supplementary Table 1). Real-time TaqMan RT–PCR was performed using the ABI Prism 7900 Detection System (Applied Biosystems) according to the manufacturer's recommended protocol. Each reaction was performed in duplicate. To determine absolute copy numbers for all p53 and p73 isoforms, standard curves were generated as reported previously (Concin et al, 2004).
Analysis of p53 mutational status
To detect alterations in the p53 gene resulting in a functionally inactive protein, a functional yeast-based assay and subsequent sequencing were performed as described in detail previously (Concin et al, 2004). Throughout the manuscript the term ‘mutant p53’ is used synonymously for ‘functionally inactivated p53’ as determined by the yeast-based assay.
Statistical analysis
The Shapiro–Wilk test was performed to assess the normality assumption. As the distribution of p53 and p73 isoforms was non-Gaussian, the Mann–Whitney U-test was performed to compare isoform expression and age with p53 mutational status. The χ2-test was performed to examine the relationship between the p53 mutational status and categorical clinicopathological parameters. For correlations among isoforms, Spearman's correlation coefficients were calculated.
Cases were divided by the 50th percentile of p53 and p73 isoform expression levels into two approximately same-sized groups (i.e., a high-expressing group and a low-expressing group of isoform expression). In addition, a TAp73/ΔTAp73 quotient was calculated and again divided by the 50th percentile. The χ2-test was used to examine the relationships between isoform expression and clinicopathological parameters.
Survival probabilities were calculated with the product limit method of Kaplan and Meier. The Cox proportional hazards model was used for multivariate analysis to assess the independence of different prognostic factors. Statistical Package for the Social Sciences for Windows 18.0 software (SPSS, Inc., Chicago, IL, USA) was used for all analyses. P-values <0.05 were considered statistically significant.
Results
Patient characteristics
In all, 154 patients diagnosed with serous ovarian cancer were enrolled. Median age was 57 years (range: 26–83). Of the patients, 81.8% (126 of 154) presented with FIGO stage III and 18.2% (28 of 154) with stage IV disease. In all, 3.9% (6 of 154) had well, 23.4% (36 of 154) moderately, and 72.7% (112 of 154) poorly differentiated carcinomas. Primary debulking surgery and subsequent platinum-based chemotherapy were performed in 87.7% (135 of 154) of the patients. In 12.3% (19 of 154) of the patients, neoadjuvant platinum-based chemotherapy was administered after diagnostic laparoscopy and interval surgery was performed. Complete macroscopic resection was achieved in 71.4% (110 of 154) of the patients. Residual disease was ⩽1 cm in 18.2% (28 of 154) and >1 cm in 10.4% (16 of 154) of the patients. Table 1 provides the prognostic relevance of clinicopathological variables in univariate and multivariate analysis.
Median follow-up was 24.5 months (range: 1–49). Of the patients, 24.8% (38 of 153) suffered treatment failure, defined as primary progression or early recurrence within 6 months after termination of primary platinum-based chemotherapy. In one patient, treatment response could not be determined due to short follow-up.
Clinical relevance of p53 mutational status
Of the ovarian cancer specimens, 78.6% (121 of 154) harboured p53 mutations and 21.4% (33 of 154) were wild-type p53. p53 mutant carcinomas were significantly associated with older age at diagnosis (median 58 vs 50 years, P<0.001) and adverse tumour grade (III vs I/II, P<0.001) compared with p53 wild-type carcinomas. Tumour stage, residual disease, and treatment response did not differ with respect to p53 mutational status (Table 2).
Patients with p53 mutant ovarian cancer showed impaired recurrence-free (mean 22.4 vs 25.7 months) and overall survival (mean 37.1 vs 39.0 months) as compared with patients with p53 wild-type cancer. Neither difference was statistically significant (P=0.223, P=0.291).
p53 and p73 isoform expression levels did not differ between p53 mutant and p53 wild-type ovarian cancer specimens. Median copy numbers stratified by p53 mutational status are provided in Supplementary Table 2.
p53 isoforms influence prognosis depending on p53 mutational status
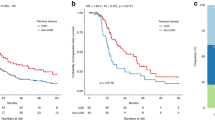
In patients with p53 mutant ovarian cancer, high Δ133p53 expression was associated with significantly improved recurrence-free and overall survival (Table 3; Figure 2A and B). A mean recurrence-free survival of 27.0 (95% CI: 22.5–31.5) months was observed in patients with high Δ133p53 expression as compared with 17.5 (95% CI: 14.6–20.4) months in patients with low Δ133p53 expression (P=0.002). Mean overall survival was 41.1 (95% CI: 37.4–44.7) months in patients with high Δ133p53 expression and 32.7 (95% CI: 28.4–37.1) months in patients with low Δ133p53 expression (P=0.007). The median survival times were not determined as <50% of patients were dead at the time of analysis, thus means are provided.
Kaplan–Meier survival graphs for p53 isoforms. High Δ133p53 expression was associated with improved recurrence-free (A) and overall survival (B) in 121 patients with p53 mutant ovarian cancer in univariate analyses. In patients with p53 wild-type cancer (n=33), high Δ40p53 expression levels predicted improved recurrence-free (C), but not overall survival (D) in univariate survival analyses. Cases were divided at the 50th percentile of p53 isoform expression levels into a high- and a low-expressing group. P-value was determined with the log-rank test.
A multivariate model comprising Δ133p53 expression and all clinicopathological parameters (age at diagnosis, tumour stage, tumour grade, and residual disease) was generated in patients with p53 mutant cancer (Table 4). Δ133p53 expression constituted an independent prognostic marker for recurrence-free (hazard ratio=0.571, P=0.016, 95% CI: 0.362–0.899) and overall survival (hazard ratio=0.365, P=0.004, 95% CI: 0.182–0.731). Moreover, tumour stage and residual disease were independently associated with recurrence-free survival (hazard ratio=2.287, P=0.001, 95% CI: 1.373–3.809 and hazard ratio=1.729, P=0.021, 95% CI: 1.085–2.753, respectively), and age at primary diagnosis with overall survival (hazard ratio=1.046, P=0.006, 95% CI: 1.013–1.079).
Δ133p53 expression correlated with response to primary treatment in patients with p53 mutant cancer. While only 18.3% (11 of 60) of the patients with high Δ133p53 expression showed treatment failure, 34.4% (21 of 61) of the patients with low Δ133p53 expression failed to respond to primary treatment. This association did not reach statistical significance (P=0.063).
In patients with p53 wild-type cancer, high Δ40p53 expression was significantly associated with improved recurrence-free (33.4 vs 19.6 months, P=0.029), but not with overall survival (43.1 vs 33.6 months, P=0.139; Table 3; Figure 2C and D). Due to small case numbers, multivariate analysis could not be performed.
In the entire group of serous ovarian cancer cases, p53 isoforms did not possess prognostic relevance. Detailed survival data are given in Supplementary Table 3.
Correlations between p53 isoforms and clinicopathological parameters
In patients with p53 mutant cancer, p53 isoform expression was not associated with clinicopathological parameters. In patients with p53 wild-type cancer, Δ40p53 expression levels correlated with favourable tumour grading (grade I/II vs III, P=0.037). In the entire group of advanced serous ovarian cancer cases, no correlation was seen between p53 isoforms and clinicopathological parameters.
Significant correlations between all p53 isoforms were observed in p53 mutant cases as well as the entire group of ovarian carcinomas, whereas the only correlation found in p53 wild-type cases was between Δ40p53 and FLp53 expression. Detailed information is provided in Supplementary Table 4.
p73 isoforms lack prognostic significance
Neither TAp73 nor ΔTAp73 expression was associated with prognosis in the examined ovarian cancer cases. Given the fact that ΔTAp73 acts in a dominant-negative manner on TAp73, we tested the hypothesis that patients might have different clinical outcomes depending on the TAp73/ΔTAp73 ratio. This ratio also did not possess prognostic relevance in the patients with advanced serous ovarian cancer. In patients with p53 wild-type ovarian cancer, TAp73/ΔTAp73 ratio correlated with favourable tumour grading (grade I/II vs III, P=0.011).
Correlations between p73 isoforms are provided in Supplementary Table 2.
Discussion
We herein provide first evidence for a potential clinical role of N-terminally truncated p53 isoforms with respect to the p53 mutational status in ovarian cancer. Only one previous study has analysed the expression of p53 isoforms in ovarian cancer (Marabese et al, 2007). Marbese et al (2007) found that FLp53 was significantly elevated in early as compared with advanced disease. However, p53 isoforms lacked prognostic significance in their group of patients with ovarian carcinomas comprising various histological subtypes.
In the present study, Δ133p53 expression constituted an independent prognostic marker in patients with p53 mutant advanced serous ovarian cancer, which represents the vast majority of ovarian cancer cases. High Δ133p53 expression resulted in a 43% risk reduction for recurrence and a 64% risk reduction for death as compared with low Δ133p53 expression. To date, only scarce information is available on the function of this N-terminally truncated p53 isoform (Jänicke et al, 2009). Existing in-vitro studies evaluated the function of Δ133p53 either alone or in the presence of wild-type p53 (Bourdon et al, 2005; Chen et al, 2005). Δ133p53 has been shown to form heterocomplexes with wild-type FLp53, resulting in the dominant-negative inhibition of FLp53 (Bourdon et al, 2005). The present clinical study, however, demonstrates a favourable role of Δ133p53 in p53 mutant carcinomas. It is unclear how Δ133p53 might exert a beneficial function in the presence of mutant p53. We hypothesise that Δ133p53 may interact with mutant p53, thereby abolishing negative effects of mutant p53. Mutant p53 has been reported to counteract wild-type FLp53 and TAp73 in a dominant-negative manner, and specific mutants possess oncogenic gain-of-function mutations (Oren and Rotter, 2010). Functional studies analysing the role of Δ133p53 in the presence of mutant p53 are highly warranted.
A possible explanation for differing Δ133p53 levels in cancer specimens observed in the present study might be provided by the presence of several polymorphisms within the internal promoter region located in the intron 4 of the p53 gene, which gives rise to Δ133p53 (Bellini et al, 2010). Bellini et al (2010) reported that these polymorphisms are associated with differences in promoter activity by changing the affinity for distinct transcription factors.
In the present study, Δ40p53 influenced recurrence-free but not overall survival in p53 wild-type ovarian cancer. We assume that the latter is related to the short follow-up period in the present study. Existing in-vitro studies suggest that Δ40p53 might enhance the function of wild-type FLp53 (Jänicke et al, 2009). Δ40p53 and FLp53 have been reported to readily form heterocomplexes (Courtois et al, 2002; Ghosh et al, 2004). As Δ40p53 lacks the MDM2-binding site, these heterocomplexes escape MDM2-mediated degradation and therefore accumulate (Yin et al, 2002). In addition, Δ40p53 supports a conformation of FLp53 that is associated with a more active state (Powell et al, 2008). Furthermore, Powell et al (2008) reported that Δ40p53 alters the post-translational modification profile of FLp53. Post-translational modifications at the N-terminus of FLp53, for instance, might increase the recruitment of transcriptional co-activators such as p300 and PCAF, and thus be responsible for increased promoter-binding capacity of the heterocomplexes. Furthermore, Δ40p53 alone has been reported to induce apoptosis through the transcriptional activation of many apoptosis-related genes that are not induced by FLp53, such as TP53BP2 (tumour protein p53 binding protein 2) and TIAL1 (TIA1 cytotoxic granule-associated RNA binding protein-like 1) (Ohki et al, 2007). However, the small number of p53 wild-type cases has to be considered when interpreting our finding.
p53 mutational status, determined with a highly sensitive yeast-based assay, did not impact prognosis in the examined patients with advanced serous ovarian cancer. In our previous retrospective study including a heterogeneous group of various histological subtypes and stages, p53 mutational status constituted a significant prognostic marker in univariate, but not in multivariate analyses (Concin et al, 2005). A recent meta-analysis evaluated the role of p53 mutational status in ovarian cancer (de Graeff et al, 2009). In studies restricted to serous carcinomas, a modest effect of p53 on overall survival was present (HR=1.61, 95% CI: 1.09–2.38). The present study agrees with the meta-analysis restricted to advanced disease, in that it found no prognostic value of p53.
Existing studies in ovarian cancer have reported inconsistent results on the clinical relevance of p73 isoforms (Buhlmann and Pützer, 2008). High levels of N-terminally truncated p73 isoforms were found to be independently associated with impaired prognosis in p53 mutant cases (Concin et al, 2005). In contrast, high ΔNp73 expression correlated with better overall survival irrespective of p53 mutational status in another study (Marabese et al, 2007). However, the present prospective study was not able to verify a clinical role of p73 isoforms in ovarian cancer.
Despite the myriad existing studies investigating the extremely complex network regulating p53 function, many details remain to be explored. The generation of multiple p53 isoforms adds an additional layer of complexity to this fascinating protein. It is increasingly being recognised that the classification of carcinomas into ‘p53 wild-type’ and ‘p53 mutant’ is an oversimplification that does not acknowledge the actual activity of the p53 pathway. Our clinical findings suggest that neglecting interactions between p53 and its N-terminally truncated isoforms might constitute an important reason for the difficulties encountered when attempting to correlate p53 with prognosis and treatment response. In addition, these complex interactions also have to be considered in therapeutic efforts aiming to directly affect p53 function, such as p53-gene therapy, vaccinating against p53, and the use of MDM2 inhibitors to activate the p53 antitumour response and cytoprotective function (Cheok et al, 2011). In future, upregulation of Δ133p53 in cancer cells could be an elegant means of improving outcome in patients with p53 mutant ovarian cancer. Thus, the tumour suppressor p53 remains a highly dynamic and rapidly expanding area of research.
Our findings underline the importance of considering the complex p53 regulatory network in clinical studies. In p53 mutant advanced serous ovarian cancers, which represent the vast majority of patients, Δ133p53 expression levels discriminate between two groups of patients with substantially different clinical outcome. High Δ133p53 expression resulted in a 43% risk reduction for recurrence and a 64% risk reduction for death as compared with low Δ133p53 expression.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Avery-Kiejda KA, Zhang XD, Adams LJ, Scott RJ, Vojtesek B, Lane DP, Hersey P (2008) Small molecular weight variants of p53 are expressed in human melanoma cells and are induced by the DNA-damaging agent cisplatin. Clin Cancer Res 14: 1659–1668
Bailey SG, Cragg MS, Townsend PA (2011) Family friction as ΔNp73 antagonises p73 and p53. Int J Biochem Cell Biol 43: 482–486
Bellini I, Pitto L, Marini MG, Porcu L, Moi P, Garritano S, Boldrini L, Rainaldi G, Fontanini G, Chiarugi M, Barale R, Gemignani F, Landi S (2010) DeltaN133p53 expression levels in relation to haplotypes of the TP53 internal promoter region. Hum Mutat 31: 456–465
Boldrup L, Bourdon JC, Coates PJ, Sjöström B, Nylander K (2007) Expression of p53 isoforms in squamous cell carcinoma of the head and neck. Eur J Cancer 43: 617–623
Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, Saville MK, Lane DP (2005) p53 isoforms can regulate p53 transcriptional activity. Genes Dev 19: 2122–2137
Buhlmann S, Pützer BM (2008) DNp73 a matter of cancer: mechanisms and clinical implications. Biochim Biophys Acta 1785: 207–216
Cannistra SA (2004) Cancer of the ovary. N Engl J Med 351: 2519–2529
Chen J, Ng SM, Chang C, Zhang Z, Bourdon JC, Lane DP, Peng J (2009) p53 isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev 23: 278–290
Chen J, Ruan H, Ng SM, Gao C, Soo HM, Wu W, Zhang Z, Wen Z, Lane DP, Peng J (2005) Loss of function of def selectively up-regulates Delta113p53 expression to arrest expansion growth of digestive organs in zebrafish. Genes Dev 19: 2900–2911
Cheok CF, Verma CS, Baselga J, Lane DP (2011) Translating p53 into the clinic. Nat Rev Clin Oncol 8: 25–37
Concin N, Becker K, Slade N, Erster S, Mueller-Holzner E, Ulmer H, Daxenbichler G, Zeimet A, Zeillinger R, Marth C, Moll UM (2004) Transdominant DeltaTAp73 isoforms are frequently up-regulated in ovarian cancer. Evidence for their role as epigenetic p53 inhibitors in vivo. Cancer Res 64: 2449–2460
Concin N, Hofstetter G, Berger A, Gehmacher A, Reimer D, Watrowski R, Tong D, Schuster E, Hefler L, Heim K, Mueller-Holzner E, Marth C, Moll UM, Zeimet AG, Zeillinger R (2005) Clinical relevance of dominant-negative p73 isoforms for responsiveness to chemotherapy and survival in ovarian cancer: evidence for a crucial p53-p73 cross-talk in vivo. Clin Cancer Res 11: 8372–8383
Courtois S, Verhaegh G, North S, Luciani MG, Lassus P, Hibner U, Oren M, Hainaut P (2002) DeltaN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene 21: 6722–6728
de Graeff P, Crijns AP, de Jong S, Boezen M, Post WJ, de Vries EG, van der Zee AG, de Bock GH (2009) Modest effect of p53, EGFR and HER-2/neu on prognosis in epithelial ovarian cancer: a meta-analysis. Br J Cancer 101: 149–159
Ebrahimi M, Boldrup L, Coates PJ, Wahlin YB, Bourdon JC, Nylander K (2008) Expression of novel p53 isoforms in oral lichen planus. Oral Oncol 44: 156–161
Ghosh A, Stewart D, Matlashewski G (2004) Regulation of human p53 activity and cell localization by alternative splicing. Mol Cell Biol 24: 7987–7997
Graupner V, Schulze-Osthoff K, Essmann F, Jänicke RU (2009) Functional characterization of p53beta and p53gamma, two isoforms of the tumor suppressor p53. Cell Cycle 8: 1238–1248
Jänicke RU, Graupner V, Budach W, Essmann F (2009) The do's and don’ts of p53 isoforms. Biol Chem 390: 951–963
Köbel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, Prentice LM, Miller D, Santos J, Swenerton K, Gilks CB, Huntsman D (2008) Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 5: e232
Liu SS, Chan KY, Cheung AN, Liao XY, Leung TW, Ngan HY (2006) Expression of deltaNp73 and TAp73alpha independently associated with radiosensitivities and prognoses in cervical squamous cell carcinoma. Clin Cancer Res 12: 3922–3927
Marabese M, Marchini S, Marrazzo E, Mariani P, Cattaneo D, Fossati R, Compagnoni A, Signorelli M, Moll UM, Codegoni AM, Broggini M (2007) Expression levels of p53 and p73 isoforms in stage I and stage III ovarian cancer. Eur J Cancer 44: 131–141
Müller M, Schilling T, Sayan AE, Kairat A, Lorenz K, Schulze-Bergkamen H, Oren M, Koch A, Tannapfel A, Stremmel W, Melino G, Krammer PH (2005) TAp73/DNp73 influences apoptotic response, chemosensitivity and prognosis in hepatocellular carcinoma. Cell Death Differ 12: 1564–1577
Murray-Zmijewski F, Lane DP, Bourdon JC (2006) p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ 13: 962–972
Ohki R, Kawase T, Ohta T, Ichikawa H, Taya Y (2007) Dissecting functional roles of p53 N-terminal transactivation domains by microarray expression analysis. Cancer Sci 98: 189–200
Oren M, Rotter V (2010) Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol 2: a001107
Powell DJ, Hrstka R, Candeias M, Bourougaa K, Vojtesek B, Fåhraeus R (2008) Stress-dependent changes in the properties of p53 complexes by the alternative translation product p53/47. Cell Cycle 7: 950–959
Reimer D, Sadr S, Wiedemair A, Stadlmann S, Concin N, Hofstetter G, Müller-Holzner E, Marth C, Zeimet AG (2007) Clinical relevance of E2F family members in ovarian cancer—an evaluation in a training set of 77 patients. Clin Cancer Res 13: 144–151
Song W, Huo SW, Lü JJ, Liu Z, Fang XL, Jin XB, Yuan MZ (2009) Expression of p53 isoforms in renal cell carcinoma. Chin Med J (Engl) 122: 921–926
Uramoto H, Sugio K, Oyama T, Nakata S, Ono K, Morita M, Funa K, Yasumoto K (2004) Expression of DNp73 predicts poor prognosis in lung cancer. Clin Cancer Res 10: 6905–6911
Vilgelm AE, Hong SM, Washington MK, Wei J, Chen H, El-Rifai W, Zaika A (2010) Characterization of DeltaNp73 expression and regulation in gastric and esophageal tumors. Oncogene 28: 5861–5868
Winter 3rd WE, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP (2007) Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25: 3621–3627
Yin Y, Stephen CW, Luciani MG, Fåhraeus R (2002) p53 Stability and activity is regulated by Mdm2-mediated induction of alternative p53 translation products. Nat Cell Biol 4: 462–467
Acknowledgements
We thank Barbara Holzer, Martina Fleischer, Julia Rössler, and Annemarie Wiedemair for their excellent technical assistance. This work was supported by the Sixth Framework Program of the European Union and Medizinischer Forschungsfond Tirol.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies the paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Hofstetter, G., Berger, A., Schuster, E. et al. Δ133p53 is an independent prognostic marker in p53 mutant advanced serous ovarian cancer. Br J Cancer 105, 1593–1599 (2011). https://doi.org/10.1038/bjc.2011.433
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2011.433
Keywords
This article is cited by
-
Amyloid-like p53 as prognostic biomarker in serous ovarian cancer—a study of the OVCAD consortium
Oncogene (2023)
-
Porcine model elucidates function of p53 isoform in carcinogenesis and reveals novel circTP53 RNA
Oncogene (2021)
-
Influence of p53 Isoform Expression on Survival in High-Grade Serous Ovarian Cancers
Scientific Reports (2019)
-
High expression of the p53 isoform γ is associated with reduced progression-free survival in uterine serous carcinoma
BMC Cancer (2018)
-
A functional interplay between Δ133p53 and ΔNp63 in promoting glycolytic metabolism to fuel cancer cell proliferation
Oncogene (2018)