Abstract
Background:
Previous studies indicate that disulfiram (DS), an anti-alcoholism drug, is cytotoxic to cancer cell lines and reverses anticancer drug resistance. Cancer stem cells (CSCs) are the major cause of chemoresistance leading to the failure of cancer chemotherapy. This study intended to examine the effect of DS on breast cancer stem cells (BCSCs).
Methods:
The effect of DS on BC cell lines and BCSCs was determined by MTT, western blot, CSCs culture and CSCs marker analysis.
Results:
Disulfiram was highly toxic to BC cell lines in vitro in a copper (Cu)-dependent manner. In Cu-containing medium (1 μ M), the IC50 concentrations of DS in BC cell lines were 200–500 nM. Disulfiram/copper significantly enhanced (3.7–15.5-fold) cytotoxicity of paclitaxel (PAC). Combination index isobologram analysis demonstrated a synergistic effect between DS/Cu and PAC. The increased Bax and Bcl2 protein expression ratio indicated that intrinsic apoptotic pathway may be involved in DS/Cu-induced apoptosis. Clonogenic assay showed DS/Cu-inhibited clonogenicity of BC cells. Mammosphere formation and the ALDH1+VE and CD24Low/CD44High CSCs population in mammospheres were significantly inhibited by exposure to DS/Cu for 24 h. Disulfiram/copper induced reactive oxygen species (ROS) generation and activated its downstream apoptosis-related cJun N-terminal kinase and p38 MAPK pathways. Meanwhile, the constitutive NFκB activity in BC cell lines was inhibited by DS/Cu.
Conclusion:
Disulfiram/copper inhibited BCSCs and enhanced cytotoxicity of PAC in BC cell lines. This may be caused by simultaneous induction of ROS and inhibition of NFκB.
Similar content being viewed by others
Main
The development of drug resistance remains the major obstacle to the success of breast cancer (BC) chemotherapy. Drug-induced DNA damage triggers the expression of anti-apoptotic proteins, which confer drug resistance upon cancer. The NFκB is one of the major chemoresistance-related anti-apoptotic factors. Many human cancers including BC possess high levels of the constitutive NFκB activity, which can be further induced by some anticancer drugs. High NFκB activity links inflammation and tumourigenesis (Greten et al, 2004). Activated NFκB triggers a series of molecular reactions including up-regulation of anti-apoptotic protein-encoding genes (Dutta et al, 2006) that induce cancer chemoresistance. High NFκB activity has been identified in drug-resistant cancer cells and ectopic over-expression of NFκB can block anticancer drug-induced apoptosis (Wang et al, 1998, 1999, 2004). Previous studies in our laboratory demonstrate that 5-fluorouracil (5-FU)- and gemcitabine (dFdC)-resistant cancer cell lines possess higher NFκB activity (Wang and Cassidy, 2003; Guo et al, 2009). Over-expression of p50 and p65, the two subunits of NFκB, results in increased NFκB activity and induces 5-FU and dFdC resistance (Wang et al, 2004; Guo et al, 2009). Although NFκB is an attractive molecular target for therapeutic intervention, inhibition of NFκB alone can only induce limited cell death. The disappointing clinical trial outcomes from using NFκB inhibitor in treatment of metastatic BC patients (Yang et al, 2006; Cresta et al, 2008) indicate that BC chemotherapy cannot be efficiently improved by only targeting NFκB pathway.
Reactive oxygen species (ROS) (Gupte and Mumper, 2009) are a group of oxygen-containing chemical species normally generated from mitochondrial respiratory chain reaction with reactive chemical properties. High ROS activity can damage DNA, protein and lipid membrane leading to apoptosis. In comparison with normal tissues, cancer cells generally possess high ROS activity (Fruehauf and Meyskens, 2007) and can tolerate higher levels of ROS. It has been suggested that further increasing ROS exposure induced by ROS-generating agents will exhaust the cellular antioxidant capacity, pushing cancer cells over the tolerated ROS threshold and leading to apoptosis (Lopez-Lazaro, 2007). Reactive oxygen species-induced apoptosis is highly reliant on persistent activation of pro-apoptotic MAPK pathways (cJun N-terminal kinases (JNKs) and p38) (Nakano et al, 2006) mainly through modulating the activities of mitochondrial pro- and anti-apoptotic proteins by phosphorylation events (Junttila et al, 2008). Many conventional anticancer drugs induce ROS generation and trigger cancer cell apoptosis via ROS–MAPK pathway. However, anticancer drug-induced ROS activation also triggers expression and activation of a number of anti-apoptotic factors including NFκB that dampen the ROS-induced cytotoxic effect (Nakano et al, 2006).
Owing to the cross-talk between NFκB and ROS–MAPK pathways, singly targeting either pathway may not be sufficient for inducing cancer cell killing. Therefore, identification of small molecules that simultaneously activate the ROS–MAPK pro-apoptotic pathway and block ROS-induced anti-apoptotic pathways may improve BC chemotherapy. Disulfiram (DS) is a commercially available anti-alcoholism drug (Johansson, 1992). We have previously demonstrated that DS inhibits NFκB activity and enhances 5-FU- and dFdC-induced apoptosis in drug-sensitive and -resistant colon cancer cell lines (Wang et al, 2003; Guo et al, 2009). Disulfiram also potentiates the cytotoxicity of cyclophosphamide, cisplatin and radiation in vitro and protects normal cells in kidney, gut and bone marrow in vivo, while increasing the therapeutic index of a wide range of cytotoxic drugs (Evans et al, 1982; Hacker et al, 1982; Bodenner et al, 1986). The molecular anticancer mechanisms of DS are still largely unknown. The previous publications indicate that the anticancer effect of DS is copper (Cu) dependent (Nobel et al, 1995; Cen et al, 2002, 2004; Chen et al, 2006). Copper has a crucial role in redox reactions and triggers generation of ROS in human cells. Disulfiram/copper is a strong ROS inducer (Nobel et al, 1995) and proteasome-NFκB pathway inhibitor (Chen et al, 2006). Combination of DS with Cu may target cancer cells by simultaneously tackling both ROS and NFκB.
Cancer derives from a very small fraction (1%) of cancer stem cells (CSCs) (Dalerba et al, 2007), which are relatively quiescent and express multidrug resistant and anti-apoptotic proteins (Marques et al, 2010; Storci et al, 2010). Conventional anticancer drugs target the proliferating and differentiated tumour bulk, but fail to eradicate the CSCs, which become the source of tumour recurrence. Aldehyde dehydrogenases (ALDHs) are functional markers of normal and breast cancer stem cells (BCSCs) (Ginestier et al, 2007; Marcato et al, 2011). It recently reported that targeting ALDH1A1 gene can target ovarian CSCs (Landen et al, 2009). Disulfiram is a specific inhibitor of ALDHs (Johansson, 1992; Lam et al, 1997). Therefore, it may also be an inhibitor of BCSCs.
This study demonstrated that in combination with physiological concentration of Cu, DS was highly cytotoxic to BCSCs and synergistically enhanced the cytotoxicity of paclitaxel (PAC) in BC cell lines.
Materials and methods
Cell lines and reagents
The BC cell lines MCF7, MDA-MB-231 and T47D were purchased from ATCC (Teddington, UK). Disulfiram, copper (II) chloride (CuCl2), N-acetyl-cysteine (NAC), SP600125 and SB203580 were purchased from Sigma (Dorset, UK).
Cell culture and cytotoxicity analysis
All cell lines were cultured in DMEM (Lonza, Wokingham, UK) supplemented with 10% FCS, 50 units ml–1 penicillin and 50 μg ml–1 streptomycin. For in vitro cytotoxicity assay, cells (5000 per well) were cultured overnight in 96-well flat-bottomed microtitre plates, then exposed to drugs for 72 h and subjected to a standard MTT assay (Plumb et al, 1989).
Analysis of the combinational effect of PAC and DS/Cu by combination index isobologram
Overnight cultured cells were exposed to various concentrations of PAC, DS+Cu1 μ M or in combination of PAC and DS+Cu1 μ M at a constant PAC:DS ratio of 62.5:1 for 72 h. The cells were then subjected to MTT analysis as described above. The combinational cytotoxicity of PAC and DS/Cu1μ M was determined using combination index (CI) isobologram analysed by CalcuSyn software (Biosoft, Cambridge, UK) (Chou and Talalay, 1984). The CI was determined by mutually exclusive equations.
Western blot analysis
Cells (80% confluence) were collected by trypsinisation and washed in ice-cold PBS and lysed in RIPA buffer. The lysate was centrifuged for 5 min in a microfuge and the supernatants retained. The primary antibodies (Cell Signaling, Herts, UK: JNK, phosphorylated JNK, cJun, phosphorylated cJun, phosphorylated p38 and cleaved PARP; Santa Cruz, CA, USA: Bcl2 and Bax) were diluted at 1:1000 in 3% BSA-TBST (anti-phosphorylated protein) or 5% fat-free milk-TBST (anti-non-phosphorylated protein). Anti-α-tubulin (Amersham, Buckinghamshire, UK; 1:8000 diluted) was used as loading control. The signal was detected using an ECL western blotting detection kit (GeneFlow, Staffordshire, UK).
Electrophoretic mobility-shift assays
Detection of NFκB-oligonucleotide complex was performed using a LightShift chemiluminescent electrophoretic mobility-shift assay kit (Pierce, Northumberland, UK). Briefly, nuclear protein (5 μg) was incubated with 20 fmol of biotin-labelled oligonucleotide for 20 min at room temperature in binding buffer. The specificity of the NFκB DNA binding was determined in competition reactions in which a 200-fold molar excess of unlabelled wild-type (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) or mutant (5′-AGT TGA TAT TAC TTT TAT AGG C-3′) NFκB probes were added to the binding reaction. The signal was detected by chemiluminescent photography.
Flow cytometric analysis of DNA content
Cells (1 × 106) were exposed to drugs and harvested by trypsinisation. The cells were fixed in 70% ethanol and then incubated with RNase A (100 μg ml–1) and propidium iodide (50 μg ml–1) for 30 min. The data from 10 000 cells of each sample were collected by FACS Scan (Becton Dickinson, Franklin Lakes, NJ, USA) and the DNA content analysed using CellQuest software (BD Biosciences, Oxford, UK).
ROS activity detection
The intracellular ROS levels were determined using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) probe (Invitrogen, Paisley, UK). Cancer cells (1 × 106) were cultured in 24-well plates with 1 ml of serum- and phenol red-free DMEM medium (Sigma) containing 20 μ M of H2DCFDA. Fluorescence was measured in 96-well plates at excitation 490 nm and emission 520 nm using a Fluoroskan Ascent fluorometer (Thermo Scientific, Northumberland, UK).
Luciferase reporter gene assay
All the transfections were performed using Lipofectamine 2000 (Invitrogen) transfection reagent following the manufacturer's instructions. The cells were co-transfected with luciferase reporter vectors (pNFκB-Tal-Luc (BD Biosciences) and pGL3-Basic (Promega, Southampton, UK)) and an internal control, pSV40-Renilla (Promega). The luciferase activities were determined using Dual Luciferase Assay reagents (Promega) following the manufacturer's instructions. The luciferase activity in each well was normalised to pSV40-Renilla using the formula of Ln=L/R (Ln, normalised luciferase activity; L, luciferase activity reading and R, Renilla activity reading). The Ln was further standardised by the transcriptional activity of the pGL3-Basic using the formula of RLU = LnNFκB/LnBasic (RLU, relative luciferase unit).
Clonogenic assay
Cells (5 × 104 per well in six-well plates) were exposed to designated concentration of DS/Cu1 μ M, PAC or PAC+DS/Cu1 μ M for 24 h. The cells were collected and further cultured for 7 (MDA-MB-231 and MCF7) to 14 (T47D) days in six-well plates containing drug-free medium at a cell density of 2.5 × 103 per well. Clonogenic cells were determined as those able to form a colony consisting of at least 50 cells.
Detection of ALDH-positive population
The ALDH-positive population in drug-treated BC cell lines was detected by ALDEFLUOR kit (StemCell Tech., Durham, NC, USA) following the supplier's instruction. The cells (2.5 × 105) were analysed after staining in ALDH substrate containing assay buffer for 30 min at 37°C. The negative control was treated with diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor.
In vitro mammosphere culture
The BC cells were cultured in ultra-low adherence six-well plates (Corning, Woburn, MA, USA) containing 2 ml of stem cell culture medium (SCM, serum-free DMEM-F12 supplemented with B27 (Invitrogen), 20 ng ml–1 epidermal growth factor (Sigma), 10 ng ml–1 basic fibroblasts growth factor (R & D System, Abingdon, UK), 10 μg ml–1 insulin (Sigma)) at a density of 10 000 cells ml–1. After 7–10 days culture, the mammospheres were photographed and subjected to further treatments.
Flow cytometric analysis of CD24 and CD44 expression
The adherent or mammosphere cells were trypsinised and passed through a 25G needle. The cells (2.5 × 105) were incubated with CD24 and CD44 antibodies (BD Pharmingen, Oxford, UK) for 20 min at 4°C. Unbound antibodies were washed off with 2% FCS HBSS (Sigma) and the cells (10 000 events) were examined no longer than 1 h after staining on a BD Facscalibur (Dorset, UK).
Statistical analysis
The data analysis was performed using Student's t-test and one-way ANOVA.
Results
The cytotoxicity of DS in BC cells was Cu dependent
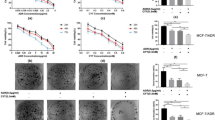
In CuCl2 (1 μ M)-supplemented medium, DS was highly cytotoxic to BC cell lines (IC50_72h: 110–476 nM; Figure 1A; Table 1). Disulfiram was also toxic to cancer cell lines in the complete medium without CuCl2 supplement with higher IC50s (456–1100 nM; Figure 1A; Table 1). A biphasic effect was observed in two out of three BC cell lines. The cancer cells appeared to be protected at higher concentrations of DS. Disulfiram alone in serum-free medium (to rule out the influence of trace amount of Cu contained in FCS) or Cu alone was not toxic to BC cell lines even at a very high concentration (20 μ M). The drug-induced morphological changes are shown in Figure 1B. The flow cytometric DNA content analysis demonstrated significant increase of apoptosis (sub-G1 population) in 72 h DS/Cu treated, but not other groups (Figure 1C). The cleaved PARP protein, an indicator of caspase activation, was detected in DS/Cu-treated cells (Figure 1D). Disulfiram/copper significantly inhibited Bcl2 and induced Bax expression; therefore, the Bax/Bcl2 ratio was increased in DS/Cu-treated cells (Figure 1E).
Disulfiram was cytotoxic to BC cells in a copper-dependent manner and synergistically enhanced cytotoxicity of PAC in BC cell lines. (A) MTT cytotoxicity assay. The BC cells were exposed to different treatments for 72 h. (B) The morphology ( × 100 magnification) of BC cell lines after 72 h drug exposure (DS: 1 μ M of DS in serum-free medium, Cu: CuCl2 1 μ M, DS/Cu: DS 1 μ M + Cu 1 μ M). (C) The DNA contents of BC cells after 72 h drug exposure (DS: 1 μ M of DS in serum-free medium, Cu: CuCl2 1 μ M, DS/Cu: DS 1 μ M+Cu 1 μ M). The DNA contents in the treated cells (10 000 events) were determined. The sub-G1 population represents the apoptotic cells (**P<0.01, n=3). The cleavage of PARP protein (D) and the expression levels of Bcl2 and Bax (E) after 72 h drug exposure were determined by western blot. Tub: α-tubulin used as a loading control. (F) MTT analysis of the combined effect of PAC and DS/Cu1 μ M. PAC:DS/Cu1 μ M=1:62.5. (G) The PAC-induced apoptosis was enhanced by DS/Cu. The DNA contents in the cell lines treated for 72 h with PAC (1 nM) or PAC plus DS/Cu (DS: MCF7, 100 nM; MDA-MB-231 and T47D, 150 nM; Cu: CuCl2 1 μ M) were determined by flow cytometry. (**P<0.01, n=3).
DS/Cu synergistically enhanced the cytotoxicity of PAC in BC cell lines
In combination with DS/Cu, the cytotoxicity of PAC was significantly enhanced in BC cell lines (4–16-fold) (Figure 1F; Table 1). There was a very strong synergistic effect between DS/Cu and PAC over a wide range of concentrations (IC50–IC90; Table 1). In contrast to the slight induction of apoptosis at low concentration of PAC alone (1 nM), the proportion of apoptotic cells was massively increased by DS/Cu (DS 100–150 nM/Cu 1 μ M) and PAC in combination (Figure 1G).
ROS activation was responsible for DS/Cu-induced cytotoxicity
The Cu-dependent cytotoxicity of DS indicates that ROS may be the mediator for DS/Cu-induced apoptosis. Disulfiram/copper significantly induced ROS activity in BC cell lines (P<0.01), which was reversed by addition of an ROS inhibitor, NAC (P<0.01; Figure 2A). To determine the effect of ROS on DS/Cu-induced cell death, the cytotoxicity assay was performed with or without ROS inhibitor. As shown in Figure 2B, the DS/Cu-induced cytotoxicity was significantly reversed by addition of NAC in the culture (P<0.01).
ROS was responsible for DS/Cu-induced cytotoxicity. (A) The BC cell lines were loaded with fluorescent dye H2DCFDA and exposed to DS5 μ M/Cu5 μ M or DS/Cu plus NAC (10 mM) for 3 h. The fluorescent strength was detected by fluorometer at Ex 490 nm and Em 520 nm. (B) The effect of ROS and MAPK pathway inhibitors on DS/Cu-induced cytotoxicity. The cancer cells were exposed to DS250 nM/Cu1 μ M, DS/Cu plus NAC (10 mM), SP600125 (10 μ M) or SB203580 (10 μ M) for 72 h and subjected to MTT assay.
DS/Cu triggered persistent activation of JNK and p38 pathways
Figure 3A shows the effect of PAC, DS/Cu and PAC/DS/Cu on the activation of the JNK pathway. Total JNK protein expression was not affected by the above treatments. However, the expression of phosphorylated JNK, cJun and total cJun was persistently (up to 24 h) induced by DS/Cu and PAC/DS/Cu. In contrast, the expression of these proteins was not or only very mildly up-regulated by PAC. High levels of phosphorylated p38 were also detected up to 24 h following DS/Cu and PAC/DS/Cu exposure (Figure 3B). To determine the causal relationship between ROS and JNK, p38 pathways, BC cell lines were exposed to DS/Cu for 24 h with or without addition of NAC. N-acetyl-cysteine significantly inhibited or totally blocked DS/Cu-induced cJun and p38 phosphorylation (Figure 3C). cJun N-terminal kinase and p38 are the major pathways responsible for ROS-induced apoptosis (McCubrey et al, 2006). Singly blocking JNK or p38 also reversed the DS/Cu-induced cytotoxicity, but at a significantly lower levels than NAC-induced ROS blocking (P<0.01; Figure 2B).
The effect of DS/Cu on MAPK and NFκB pathways. The overnight cultured BC cells were exposed to PAC1 μ M, DS1 μ M/Cu1 μ M or PAC1 μ M/DS1 μ M/Cu1 μ M for indicated time lengths. The expression levels and phosphorylation status of proteins in JNK (A) and p38 (B) pathways were detected by western blot. (C) The activation of JNK and p38 pathways was reversed by NAC. The phosphorylation of cJun and p38 in BC cell lines was determined by western blot after exposure to DS/Cu or DS/Cu plus NAC (10 mM) for 24 h. (D) NFκB DNA-binding activity was analysed by electrophoretic mobility-shift assay assay. Nu: western blot of nucleolin was used as a protein loading control. The BC cell lines were treated with PAC1 μ M, DS1 μ M/Cu1 μ M or PAC/DS/Cu for 24 h. (E) NFκB transcriptional activity examined by luciferase reporter gene assay after exposure to PAC1 μ M, DS1 μ M/Cu1 μ M or PAC/DS/Cu for 24 h.
DS/Cu inhibited NFκB activity in BC cell lines
The NFκB is an ROS-induced transcription factor with strong anti-apoptotic activity, which in turn dampens the pro-apoptotic effect of ROS (Nakano et al, 2006). Blockage of NFκB activation enhances ROS-induced cytotoxicity. Both PAC and DS/Cu inhibited NFκB DNA-binding activity in BC cell lines. The strongest inhibition was observed in the cells treated with PAC/DS/Cu in combination (Figure 3D). The inhibition of NFκB transcriptional activity was also detected in PAC-, DS/Cu- and PAC/DS/Cu-treated cells by reporter gene assay (Figure 3E).
DS/Cu inhibited the clonogenity in BC cell lines
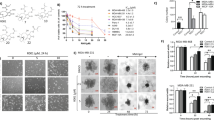
Clonogenic assays (Franken et al, 2006) were performed to examine the ability of DS/Cu to induce ‘reproductive death’ in BC cells. After 16 h exposure to PAC (40 nM: 4–18-fold higher than IC50 concentration), DS (200–250 nM: sub-IC50 concentration)/Cu1 μ M or PAC and DS/Cu in combination, the treated cells were collected and cultured in drug-free medium for 7–14 days. The colony number was reduced by exposure to PAC, DS or Cu alone. The colony number in PAC-, DS- and Cu-treated groups was decreased, which was caused by slow growth of the surviving cells leading to the cell number in some colonies not reaching the counting threshold (50 cells). In contrast, the clonogenicity of BC cell lines was significantly inhibited by DS/Cu and totally eradicated by exposure to PAC plus DS/Cu (Figure 4A).
The effect of DS/Cu on the clonogenicity and CSCs in BC cell lines. (A) Clonogenic assay. The cells exposed to Cu1 μ M, PAC40 nM, DS (250 nM for MDA-MB-231 and T47D, 200 nM for MCF7), DS/Cu or PAC/DS/Cu for 24 h were cultured in drug-free medium in six-well plates at a cell density of 2.5 × 103 per well for 7–10 days. The colonies were stained with crystal violet, counted and photographed as described in Materials and Methods. (B) DS/Cu and PAC/DS/Cu inhibited mammosphere formation. The BC cells were treated with PAC40 nM, DS250 nM, Cu1 μ M, DS/Cu or PAC/DS/Cu for 48 h and then sub-cultured in drug-free SCM in ultra-low attachment six-well plates (5000 cells per well) for 7 days and photographed at × 40 magnification. (C) DS/Cu inhibited ALDH expression in mammospheres. The ALDH+VE population was flow cytometrically determined in mammospheres exposed to drugs (Cu1 μ M, DS1 μ M or DS1 μ M/Cu1 μ M) for 16 h. (D) DS/Cu abolished CD24Low/CD44High population in mammospheres. The expression of CD24 and CD44 was examined after 16 h exposure to Cu1 μ M, DS1 μ M, PAC100 nM or DS/Cu/PAC. The inserted numbers in the frame represent percentage of ALDH+VE or CD24Low/CD44High cells (mean±s.d. from three experiments, **P<0.01).
DS/Cu targeted BCSCs
Furthermore, we examined the effect of different treatments on CSCs population in MDA-MB-231 and T47D cell lines. The mammosphere formation in both cell lines was completely blocked by exposure to DS1 μ M/Cu1 μ M or DS/Cu plus PAC40 nM for 48 h, but not affected by PAC, DS or Cu alone (Figure 4B). To determine the targeting effect of DS/Cu on CSCs, the BCSCs markers in drug-treated mammosphere cells were also analysed. Figure 4C demonstrates that the ALDH-positive population in mammospheres was significantly inhibited by DS/Cu, but not affected or even enriched by DS or Cu treatment. In order to determine the effect of DS/Cu on CSCs, the expression status of CD24Low/CD44High, another marker of BCSCs, was also examined. After 16 h exposure to different drugs, the CD24Low/CD44High population in the mammosphere cells was determined by flow cytometry. In comparison with the attached cells, the mammosphere population contained significantly higher percentage of CD24Low/CD44High BCSCs (Figure 4D). The percentage of CD24Low/CD44High cells in mammosphere was significantly reduced following 16 h exposure to DS/Cu and PAC/DS/Cu, but not influenced by PAC, DS or Cu (Figure 4D).
Discussion
Disulfiram is a Food and Drug Administration-approved anti-alcoholism drug used in clinic with extensive available pre-clinical and clinical data (Eneanya et al, 1981). Our study demonstrated high cytotoxicity of DS to BC cell lines in a Cu-dependent manner. Using Cu to treat cancer has a long history (Hieger, 1926; Gupte and Mumper, 2009), but the intracellular transport of Cu is still one of the major hurdles for its clinical efficacy. The transport of Cu into cell is mediated and tightly controlled by the copper transporter, Ctr1. A derivative of DS, N,N-diethyldithiocarbamate (deDTC), binds to Cu forming a Cu(deDTC)2 complex, which improves the intracellular trafficking of Cu and this is probably responsible for DS-induced apoptosis (Cen et al, 2004). Disulfiram can also penetrate into cancer cells to form Cu(deDTC)2 with intracellular Cu. In comparison with normal tissues, many cancers including BC possess higher levels of Cu (two- to three-fold) (Mulay et al, 1971; Margalioth et al, 1983; Rizk and Sky-Peck, 1984), which may enable DS to target cancer cells selectively (Evans et al, 1982; Hacker et al, 1982; Bodenner et al, 1986; Chen et al, 2006; Iljin et al, 2009). In line with a previous report (Wickstrom et al, 2007), a biphasic cytotoxic effect of DS was observed in BC cell lines tested in complete medium without Cu supplement (Figure 1A). Breast cancer cells were killed at low concentration, but revived at higher DS concentrations (∼10 μ M). The mechanism of the biphasic effect remains unclear. A degradation product of DS may compete trace amounts of Cu, block formation of Cu(deDTC)2 and inhibit transport of Cu into cells (Cen et al, 2004). We have previously reported that DS enhances the cytotoxicity of 5-FU and gemcitabine in colon and BC cell lines (Wang et al, 2003; Guo et al, 2009). Here, we demonstrated synergistic cytotoxic effect of DS/Cu and PAC on BC cell lines.
Previous studies demonstrate that in combination with Cu, DS induces ROS activity in melanoma cell lines (Cen et al, 2002; Morrison et al, 2010). The recent study from Dou's group demonstrates that gold–dithiocarbamato complexes strongly induce ROS and inhibit proteasome activity in BC cells (Zhang et al, 2010). In consistence with these results, our study showed that DS/Cu induced ROS activity, which was responsible for DS/Cu-induced cytotoxicity in BC cell lines. The ROS-induced apoptosis is commonly mediated by the persistent activation of JNK and p38 MAPK pathways (Junttila et al, 2008). In our study, both JNK and p38 pathways were persistently (over 24 h) activated (phosphorylation of cJun and p38) by DS/Cu and blocked by NAC. cJun N-terminal kinase and p38 inhibitors reduced cytotoxicity of DS/Cu, although to a lesser degree than ROS inhibition. Therefore, ROS-activated JNK and p38 pathways were, at least partially, responsible for ROS-induced apoptosis. The persistent activation of JNK and p38 induces apoptosis via mitochondrial apoptotic pathways (Junttila et al, 2008). The DS/Cu-induced apoptosis was confirmed by DNA content and PARP protein cleavage. The expression of Bax and Bcl2 proteins was induced and suppressed by DS/Cu, respectively, leading to increased Bax/Bcl2 ratio. The elevated Bax/Bcl2 ratio indicated that the intrinsic apoptotic pathway may be involved in DS/Cu-induced apoptosis.
Owing to the high proliferative rate and energy requirement, cancer cells are under higher ROS stress than their normal counterparts. High levels of ROS can damage DNA, mitochondrial inner membrane and membrane phospholipids leading to apoptosis (Gupte and Mumper, 2009). However, ROS also activate a wide range of anti-apoptotic factors. The effect of ROS on cancer cells depends on the balance between ROS-induced pro- and anti-apoptotic factors. The NFκB is one of the most important ROS-induced anti-apoptotic factors (Gloire et al, 2006). The NFκB activation in turn inhibits ROS and JNK, p38 activation and ultimately inhibits ROS-induced apoptosis. Breast cancer cell lines commonly possess high levels of constitutive NFκB activity (Wang et al, 2004; Guo et al, 2009; Xu et al, 2009). Consistent with previous publications (Wang et al, 2003; Guo et al, 2009), DS/Cu inhibited NFκB activity in BC cell lines. This indicates that DS/Cu may induce apoptosis of BC cells by simultaneously inducing ROS generation and inhibiting ROS-NFκB pathway.
The effect of DS/Cu on the regeneration of minimal-residual cancer cells, the main source of cancer relapse after chemotherapy, was examined using a clonogenic assay, a gold measure to detect the cell ‘reproductive death’ after cytotoxic agent treatments (Franken et al, 2006). In contrast to the moderate inhibiting effect of PAC, DS and Cu on clonogenicity of BC cells, the colony formation was significantly reduced or completely eradicated by DS/Cu and PAC/DS/Cu, respectively (Figure 4A).
Disulfiram/copper reverses cancer cell chemoresistance induced by a wide range of different mechanisms (Wang et al, 2001, 2003, 2004; Guo et al, 2009). It has been widely accepted that CSCs are responsible for tumour recurrence and may display significant resistance to different cytotoxic drugs (Liu and Wicha, 2010). The effect of DS/Cu on clonogenicity of BC cell lines prompted us to examine the effect of DS/Cu on BCSCs. Disulfiram is an inhibitor of ALDHs. Human ALDHs are a superfamily with 19 members involved in detoxifying a wide range of aldehydes to their corresponding weak carboxylic acids (Sladek, 2003); ALDH1A1 has been identified as a functional marker of several different types of CSCs including BCSCs (Ginestier et al, 2007; Alison et al, 2011). It recently reported that knockdown of ALDH1A1 expression using siRNA can target ovarian CSCs and potentiate cytotoxicity of taxane and platinum in vitro and in vivo (Landen et al, 2009). Recently, Marcato et al (2011) identify ALDH1A3 as another major marker of BCSCs. Therefore, ALDHs may be redundantly expressed in different cancer types and targeting one isoform may not be sufficient for CSCs targeting. Disulfiram is a strong inhibitor for both cytosol and mitochondrial ALDHs (Eneanya et al, 1981; Lam et al, 1997). Kast and Belda-Iniesta (2009) hypothesised that targeting ALDHs by DS may reverse chemoresistance in glioblastoma. Our study is the first report of using DS to target BCSCs. The ALDH+VE population in BCSCs was significantly inhibited by DS/Cu. The ability of BC cell lines to form mammospheres was completely inhibited by 24 h exposure to DS/Cu or PAC/DS/Cu (Figure 4B). The effect of DS/Cu on CSCs was also confirmed by the reduction of the CD24Low/CD44High population (Figures 4C and D). The detailed molecular mechanisms underlying the effect of DS/Cu on BCSCs are unclear. Aldehyde dehydrogenases detoxify intracellular aldehydes, which can form adducts with glutathione, nucleic acids and amino acids leading to cell death (Marchitti et al, 2008). The high expression of ALDHs in CSCs may be protective. Mammalian cornea cells contain abundant ALDH, which has critical role in scavenging ROS and reduce UV-induced oxidative stress (Estey et al, 2007). Aldehyde dehydrogenase deficiency in central nervous system is associated with progressive neurodegeneration (Marchitti et al, 2007). Inhibition of NFκB pathway and induction of ROS result in reduction of stem-like properties in CSCs derived from pancreatic cancer and leukaemia (Greten et al, 2004; Jin et al, 2010; Rausch et al, 2010). Disulfiram/copper may target BCSCs by simultaneously inhibiting NFκB and activating ROS activity.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Alison MR, Guppy NJ, Lim SM, Nicholson LJ (2011) Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose? J Pathol 222: 335–344
Bodenner DL, Dedon PC, Keng PC, Katz JC, Borch RF (1986) Selective protection against cis-diamminedichloroplatinum(II)-induced toxicity in kidney, gut, and bone marrow by diethyldithiocarbamate. Cancer Res 46: 2751–2755
Cen D, Brayton D, Shahandeh B, Meyskens Jr FL, Farmer PJ (2004) Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem 47: 6914–6920
Cen D, Gonzalez RI, Buckmeier JA, Kahlon RS, Tohidian NB, Meyskens Jr FL (2002) Disulfiram induces apoptosis in human melanoma cells: a redox-related process. Mol Cancer Ther 1: 197–204
Chen D, Cui QC, Yang H, Dou QP (2006) Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res 66: 10425–10433
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22: 27–55
Cresta S, Sessa C, Catapano CV, Gallerani E, Passalacqua D, Rinaldi A, Bertoni F, Vigano L, Maur M, Capri G, Maccioni E, Tosi D, Gianni L (2008) Phase I study of bortezomib with weekly paclitaxel in patients with advanced solid tumours. Eur J Cancer 44: 1829–1834
Dalerba P, Cho RW, Clarke MF (2007) Cancer stem cells: models and concepts. Annu Rev Med 58: 267–284
Dutta J, Fan Y, Gupta N, Fan G, Gelinas C (2006) Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 25: 6800–6816
Eneanya DI, Bianchine JR, Duran DO, Andresen BD (1981) The actions of metabolic fate of disulfiram. Annu Rev Pharmacol Toxicol 21: 575–596
Estey T, Piatigorsky J, Lassen N, Vasiliou V (2007) ALDH3A1: a corneal crystallin with diverse functions. Exp Eye Res 84: 3–12
Evans RG, Engel C, Wheatley C, Nielsen J (1982) Modification of the sensitivity and repair of potentially lethal damage by diethyldithiocarbamate during and following exposure of plateau-phase cultures of mammalian cells to radiation and cis-diamminedichloroplatinum(II). Cancer Res 42: 3074–3078
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1: 2315–2319
Fruehauf JP, Meyskens Jr FL (2007) Reactive oxygen species: a breath of life or death? Clin Cancer Res 13: 789–794
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1: 555–567
Gloire G, Legrand-Poels S, Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72: 1493–1505
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M (2004) IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118: 285–296
Guo X, Xu B, Pandey S, Goessl E, Brown J, Armesilla AL, Darling JL, Wang W (2009) Disulfiram/copper complex inhibiting NFkappaB activity and potentiating cytotoxic effect of gemcitabine on colon and breast cancer cell lines. Cancer Lett 290: 104–113
Gupte A, Mumper RJ (2009) Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev 35: 32–46
Hacker MP, Ershler WB, Newman RA, Gamelli RL (1982) Effect of disulfiram (tetraethylthiuram disulfide) and diethyldithiocarbamate on the bladder toxicity and antitumor activity of cyclophosphamide in mice. Cancer Res 42: 4490–4494
Hieger I (1926) The effect of copper compounds upon the growth of carcinoma in the rat. Biochem J 20: 232–236
Iljin K, Ketola K, Vainio P, Halonen P, Kohonen P, Fey V, Grafstrom RC, Perala M, Kallioniemi O (2009) High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin Cancer Res 15: 6070–6078
Jin Y, Lu Z, Ding K, Li J, Du X, Chen C, Sun X, Wu Y, Zhou J, Pan J (2010) Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res 70: 2516–2527
Johansson B (1992) A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand Suppl 369: 15–26
Junttila MR, Li SP, Westermarck J (2008) Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J 22: 954–965
Kast RE, Belda-Iniesta C (2009) Suppressing glioblastoma stem cell function by aldehyde dehydrogenase inhibition with chloramphenicol or disulfiram as a new treatment adjunct: an hypothesis. Curr Stem Cell Res Ther 4: 314–317
Lam JP, Mays DC, Lipsky JJ (1997) Inhibition of recombinant human mitochondrial and cytosolic aldehyde dehydrogenases by two candidates for the active metabolites of disulfiram. Biochemistry 36: 13748–13754
Landen Jr CN, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast Jr RC, Coleman RL, Lopez-Berestein G, Sood AK (2009) Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther 9: 3186–3199
Liu S, Wicha MS (2010) Targeting breast cancer stem cells. J Clin Oncol 28: 4006–4012
Lopez-Lazaro M (2007) Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett 252: 1–8
Marcato P, Dean CA, Pan D, Araslanova R, Gillis M, Joshi M, Helyer L, Pan L, Leidal A, Gujar S, Giacomantonio CA, Lee PW (2011) Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells 29: 32–45
Marchitti SA, Deitrich RA, Vasiliou V (2007) Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol Rev 59: 125–150
Marchitti SA, Brocker C, Stagos D Vasiliou V (2008) Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol 4: 697–720
Margalioth EJ, Schenker JG, Chevion M (1983) Copper and zinc levels in normal and malignant tissues. Cancer 52: 868–872
Marques DS, Sandrini JZ, Boyle RT, Marins LF, Trindade GS (2010) Relationships between multidrug resistance (MDR) and stem cell markers in human chronic myeloid leukemia cell lines. Leuk Res 34: 757–762
McCubrey JA, Lahair MM, Franklin RA (2006) Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 8: 1775–1789
Morrison BW, Doudican NA, Patel KR, Orlow SJ (2010) Disulfiram induces copper-dependent stimulation of reactive oxygen species and activation of the extrinsic apoptotic pathway in melanoma. Melanoma Res 20: 11–20
Mulay IL, Roy R, Knox BE, Suhr NH, Delaney WE (1971) Trace-metal analysis of cancerous and noncancerous human tissues. J Natl Cancer Inst 47: 1–13
Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K (2006) Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ 13: 730–737
Nobel CI, Kimland M, Lind B, Orrenius S, Slater AF (1995) Dithiocarbamates induce apoptosis in thymocytes by raising the intracellular level of redox-active copper. J Biol Chem 270: 26202–26208
Plumb JA, Milroy R, Kaye SB (1989) Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res 49: 4435–4440
Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, Wirth T, Schemmer P, Buchler MW, Zoller M, Salnikov AV, Herr I (2010) Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res 70: 5004–5013
Rizk SL, Sky-Peck HH (1984) Comparison between concentrations of trace elements in normal and neoplastic human breast tissue. Cancer Res 44: 5390–5394
Sladek NE (2003) Human aldehyde dehydrogenases: potential pathological, pharmacological, and toxicological impact. J Biochem Mol Toxicol 17: 7–23
Storci G, Sansone P, Mari S, D'Uva G, Tavolari S, Guarnieri T, Taffurelli M, Ceccarelli C, Santini D, Chieco P, Marcu KB, Bonafe M (2010) TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype. J Cell Physiol 225: 682–691
Wang CY, Cusack Jr JC, Liu R, Baldwin Jr AS (1999) Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med 5: 412–417
Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin Jr AS (1998) NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281: 1680–1683
Wang W, Cassidy J (2003) Constitutive nuclear factor-kappa B mRNA, protein overexpression and enhanced DNA-binding activity in thymidylate synthase inhibitor-resistant tumour cells. Br J Cancer 88: 624–629
Wang W, Cassidy J, O'Brien V, Ryan KM, Collie-Duguid E (2004) Mechanistic and predictive profiling of 5-fluorouracil resistance in human cancer cells. Cancer Res 64: 8167–8176
Wang W, Marsh S, Cassidy J, McLeod HL (2001) Pharmacogenomic dissection of resistance to thymidylate synthase inhibitors. Cancer Res 61: 5505–5510
Wang W, McLeod HL, Cassidy J (2003) Disulfiram-mediated inhibition of NF-kappaB activity enhances cytotoxicity of 5-fluorouracil in human colorectal cancer cell lines. Int J Cancer 104: 504–511
Wickstrom M, Danielsson K, Rickardson L, Gullbo J, Nygren P, Isaksson A, Larsson R, Lovborg H (2007) Pharmacological profiling of disulfiram using human tumor cell lines and human tumor cells from patients. Biochem Pharmacol 73: 25–33
Xu B, Guo X, Mathew S, Armesilla AL, Cassidy J, Darling JL, Wang W (2009) Triptolide simultaneously induces reactive oxygen species, inhibits NF-kappaB activity and sensitizes 5-fluorouracil in colorectal cancer cell lines. Cancer Lett 291: 200–208
Yang CH, Gonzalez-Angulo AM, Reuben JM, Booser DJ, Pusztai L, Krishnamurthy S, Esseltine D, Stec J, Broglio KR, Islam R, Hortobagyi GN, Cristofanilli M (2006) Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol 17: 813–817
Zhang X, Frezza M, Milacic V, Ronconi L, Fan Y, Bi C, Fregona D, Dou QP (2010) Inhibition of tumor proteasome activity by gold-dithiocarbamato complexes via both redox-dependent and -independent processes. J Cell Biochem 109: 162–172
Acknowledgements
This work is supported by Breast Cancer Campaign, UK. P Liu is a PhD student supported by RIHS and Deten Pharmaceutical Ltd, Xi'an, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yip, N., Fombon, I., Liu, P. et al. Disulfiram modulated ROS–MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer 104, 1564–1574 (2011). https://doi.org/10.1038/bjc.2011.126
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2011.126
Keywords
This article is cited by
-
Augmenting MEK inhibitor efficacy in BRAF wild-type melanoma: synergistic effects of disulfiram combination therapy
Journal of Experimental & Clinical Cancer Research (2024)
-
Development and Evaluation of PLGA Nanoparticles Surfaced Modified with Chitosan-Folic Acid for Improved Delivery of Resveratrol to Prostate Cancer Cells
BioNanoScience (2024)
-
FDA-approved disulfiram as a novel treatment for aggressive leukemia
Journal of Molecular Medicine (2024)
-
Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies
Cell Death & Disease (2023)
-
The SP/NK1R system promotes the proliferation of breast cancer cells through NF-κB-mediated inflammatory responses
Cell Biochemistry and Biophysics (2023)