Abstract
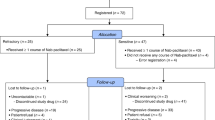
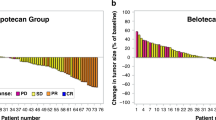
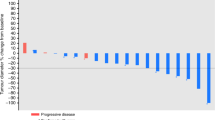
In a multicentre trial of the EORTC ECTG we have treated 43 non-pretreated patients with advanced non-small-cell lung cancer (NSCLC) with the new semisynthetic taxoid docetaxel (Taxotere). Six patients were ineligible; of the 37 eligible patients, ten had prior radiotherapy and 18 prior surgery. They received 100 mg m-2 in 1 h i.v. every 3 weeks, usually in an outpatient setting. Prophylactic steroids, antihistaminics or antiemetics were not routinely given. Two patients were not evaluable because they withdrew from the study because of a hypersensitivity reaction after the second cycle. The main toxicity was neutropenia (80% of cycles), although infections were rare (4%). One patient died from sepsis during neutropenia. Hypersensitivity reactions necessitating interruption of docetaxel (Taxotere) infusions were found in only 10% of cycles. The overall response rate was 23% with one complete response, and seven partial responses. Stable disease was found in 16 patients. The median duration of response was 36 weeks, and the median survival of all patients was 11 months. Docetaxel (Taxotere) is among the most active drugs for treatment of NSCLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Cerny, T., Kaplan, S., Pavlidis, N. et al. Docetaxel (TaxotereTM) is active in non-small-cell lung cancer: a phase II trial of the EORTC early clinical trials group (ECTG). Br J Cancer 70, 384–387 (1994). https://doi.org/10.1038/bjc.1994.311
Issue Date:
DOI: https://doi.org/10.1038/bjc.1994.311
This article is cited by
-
A simple method to improve the stability of docetaxel micelles
Scientific Reports (2016)
-
Role of tumor necrosis factor alpha-induced protein 1 in paclitaxel resistance
Oncogene (2014)
-
Phase II clinical trial with gemcitabine and paclitaxel sequential monotherapy as first-line treatment for advanced non-small-cell lung cancer (SLCG 01-04)
Clinical and Translational Oncology (2011)
-
Phase II study of carboplatin combined with weekly docetaxel in patients with advanced non-small cell lung cancer
Cancer Chemotherapy and Pharmacology (2010)
-
Phase II study with fractionated schedule of docetaxel and cisplatin in patients with advanced non-small cell lung cancer
Cancer Chemotherapy and Pharmacology (2010)