Abstract
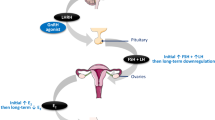
Thirty patients with advanced epithelial ovarian cancer were treated with the luteinising hormone releasing agonist, goserelin. There were two partial responses lasting 40 and 105 weeks respectively. In addition five patients had disease stabilisation lasting 25, 35, 40, 66 and 70 weeks respectively and 23 patients had progressive disease. No significant or unexpected toxicities occurred. This minimally toxic therapy halted disease progression for 6 months or more in 23% of patients, the majority of whom were heavily pretreated. There were five early deaths due to disease progression. The use of goserelin in patients with epithelial ovarian cancers resistant to or relapsing soon after first line platinum based chemotherapy needs to be further evaluated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lind, M., Cantwell, B., Millward, M. et al. A phase II trial of goserelin (Zoladex) in relapsed epithelial ovarian cancer. Br J Cancer 65, 621–623 (1992). https://doi.org/10.1038/bjc.1992.126
Issue Date:
DOI: https://doi.org/10.1038/bjc.1992.126
This article is cited by
-
Phase II trial of tamoxifen and goserelin in recurrent epithelial ovarian cancer
British Journal of Cancer (2005)