Abstract
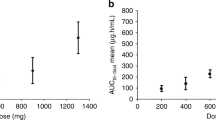
A phase 1 study of a new ribonucleotide reductase inhibitor, didox, was performed by administration of escalating doses of the drug by slow i.v. injection. Thirty-four patients with unresponsive metastatic carcinoma received the drug. There were 13 escalations of dosage, from a starting dose of 192 mg m-2 to 10 g m-2. Dose limiting toxicity was encountered at 7.5 g m-2 where disturbances of hepatic and renal function were observed, in addition to severe gastrointestinal toxicity. Pharmacokinetic studies showed that a peak level of didox was achieved within 5 minutes of injection. At 1,728 mg m-2 the data best fitted a 2 compartment open model, with a mean serum alpha t1/2 of 5.2 min, with a beta t1/2 of 41.3 min. Less than 10% of the drug was excreted unchanged in the urine and the majority of this excretion was within 6 h. Didox can therefore be safely given by slow i.v. injection at a dose of 6 g m-2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Veale, D., Carmichael, J., Cantwell, B. et al. A phase 1 and pharmacokinetic study of didox: A ribonucleotide reductase inhibitor. Br J Cancer 58, 70–72 (1988). https://doi.org/10.1038/bjc.1988.164
Issue Date:
DOI: https://doi.org/10.1038/bjc.1988.164
This article is cited by
-
Clinical pharmacology and clinical trials of ribonucleotide reductase inhibitors: is it a viable cancer therapy?
Journal of Cancer Research and Clinical Oncology (2017)
-
Evaluation of rodent-only toxicology for early clinical trials with novel cancer therapeutics
British Journal of Cancer (1999)
-
Biochemical and antitumor activity of trimidox, a new inhibitor of ribonucleotide reductase
Cancer Chemotherapy and Pharmacology (1994)
-
Growth, ribonucleotide reductase and metals in murine leukemic lymphocytes
Journal of Cancer Research and Clinical Oncology (1991)
-
Cisplatin with high-dose infusions of hydroxyurea to inhibit DNA repair
Cancer Chemotherapy and Pharmacology (1989)