Abstract
Aims/Objectives:
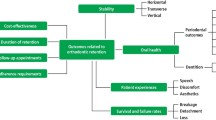
To evaluate studies of the effectiveness of different tooth replacement strategies in adult patients with shortened dental arches (SDA). Specifically, the objectives of the proposed review are to determine the survival rates of different prosthodontic interventions; the risk of tooth loss with and without different prosthodontic interventions; and the impact of different tooth replacement strategies on oral-health related quality of life (OHRQoL).
Materials and methods:
The protocol has been registered with the International Prospective Register of Systematic Reviews (PROSPERO), and was developed in accordance with the guidelines of the Preferred Reporting Items for Systematic Review and Meta-analyses Protocols (PRISMA-P). Studies will be selected according to outlined eligibility criteria including types of studies, participants, interventions, comparators and outcomes. Specific search strategies will be created and data collection and analysis will be undertaken by two independent reviewers.
Discussion:
The review will assess the body of evidence for clinical decision making in patients with SDA and reduced dentitions, by comparing the effectiveness of different tooth replacement strategies. In addition, it will assess the influence of patients in this decision making, help to inform subsequent cost-effectiveness analyses, identify areas of further research and hopefully inform future healthcare policy.
Similar content being viewed by others
Introduction
The population of the world is ageing. The United Nations has estimated that globally, the proportion of older persons (60 years and over) increased from 9.2% in 1990 to 11.7% in 2013. It is expected that this proportion will rise to 21.1% by 2050, with an elderly population of more than 2 billion.1 As significant transformations are occurring in populations, changes have also been noted in oral health. More and more adults are retaining their natural teeth into old age. The 2009 UK Adult Dental Health Survey (ADHS) reported that only 6% of those surveyed were missing all their teeth, a significant decrease from 37% in 1968.2 With increased tooth retention, population growth and aging, the global burden of oral conditions has increased by ~20.8% since 1990. Collectively, oral conditions affected 3.9 billion people worldwide in 2010.3
Potential consequences of tooth loss include impaired mastication, altered food choices, psychosocial problems and reduced oral health-related quality of life.4,5 However, depending on the pattern of tooth loss, it may not be necessary to replace missing teeth, especially in older patients. Kayser6 first described the shortened dental arch (SDA) concept, suggesting that patients with at least four occlusal units (one unit=pair of occluding premolars; two units=pair of occluding molars) had sufficient adaptive capacity to constitute a functional dentition. The concept has been suggested as an oral health goal for adults until the end of life by the World Health Organisation,7 and is considered to have a useful role in contemporary clinical practice.8
Where tooth replacement is required to restore partially dentate patients to at least a reduced functional dentition, there are various fixed and removable prosthetic options. Traditionally these have included removable partial dentures, and resin bonded or conventional bridgework. In the last number of decades these options have grown in scope with the demonstrated predictability of dental implants. However, decision making for different patterns of tooth loss and patient groups is often not evidence based.9 In addition, the financial cost of tooth loss disproportionately affects older age groups,10 and there is a need to achieve better clinical outcomes, which are also cost-effective.
Therefore, the aim of this systematic review is to evaluate studies of the effectiveness of different tooth replacement strategies in adult patients with shortened dental arches. Specifically, the objectives of the proposed review are to determine the:
-
survival rates of different prosthodontic interventions;
-
risk of tooth loss with and without different prosthodontic interventions; and
-
impact of different tooth replacement strategies on oral-health related quality of life (OHRQoL).
Materials and methods
This protocol has been registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42017064851), and developed in accordance with the guidelines of the Preferred Reporting Items for Systematic Review and Meta-analyses Protocols (PRISMA-P).11
Eligibility criteria
Studies will be selected according to the criteria outlined below
Types of studies
We will include studies that used an experimental or observational design:
-
randomized controlled trials (RCTs);
-
cluster RCTs;
-
non-randomized controlled trials (NRCTs);
-
controlled before after studies (CBAs); and
-
interrupted time series studies (ITSs).
An initial scoping review and the expertise of the review team suggest that it is appropriate to include observational studies as relatively few randomized trials have addressed questions about the effects of interventions.
Types of participants
Partially dentate male and female adults (18 years or older) will be included. Individuals must have between 4 and 10 functional maxillary and/or mandibular teeth to be eligible.
Types of interventions
The following prosthodontic interventions will be eligible:
-
Acrylic or metal-based removable partial dentures (including those with precision attachments)
-
Conventional or resin bonded bridgework (including cantilever and fixed–fixed designs)
-
Implant supported crown or bridgework
The comparator will be no intervention or different interventions (‘head-to-head’).
Types of outcomes
Primary outcomes
The primary outcomes of the effectiveness of prosthodontic interventions will be measured in terms of:
-
survival of prosthodontic interventions (mean follow-up of 5 years or more);
-
survival of remaining teeth (mean follow-up of 5 years or more); and
-
change in OHRQoL using validated self-reported measures (mean follow-up of 1 year or more).
Secondary outcomes
We will collect other relevant outcome measures including the following:
-
Biological complications (dental caries, periodontal/peri-implant disease, loss of tooth vitality, coronal/root fracture)
-
Technical complications (loss of retention, fracture/deformation, abutment/screw fracture/loosening)
Search methods for identification of studies
Electronic searches
In consultation with the authors, specific search strategies will be created by a health services librarian with expertise in systematic reviewing. A copy of the MEDLINE search strategy is available in Figure 1. We will adapt the MEDLINE strategy for other databases and translate MeSH terms to the controlled vocabularies of those databases as appropriate. We will publish all search strategies used in the review. All databases will be searched from inception to the date of the search.
We will search the following databases for primary studies:
-
MEDLINE, 1980 to present, in-process and other non-indexed citations, Ovid SP
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
Embase, 1980 to present, Ovid SP
We will search the following trial registries:
-
International Clinical Trials Registry Platform (ICTRP), World Health Organization (WHO)
-
ClinicalTrials.gov, US National Institutes of Health (NIH)
We will also conduct a grey literature search, using the OpenSIGLE database, to identify studies not indexed in the databases listed above.
Searching other resources
To ensure literature saturation, we will review reference lists of included studies or reviews identified through the search. Where necessary, we will also contact the authors of eligible studies and researchers with expertise relevant to the review topic. Authors of relevant studies will be contacted and asked to provide additional data relevant to the review. All strategies used, including a list of sources screened and relevant reviews/primary studies reviewed, will be provided in appendices.
Data collection and analysis
Selection of studies
Two independent reviewers (CML and CM) will screen the titles and abstracts identified by the electronic searches in duplicate. Full reports will be obtained for all titles that appear to meet the inclusion criteria or where there is uncertainty. Additional information from study authors will be requested where necessary. Disagreements between reviewers will be resolved by discussion, and one of two arbitrators (GMK or MD) will adjudicate unresolved disagreements. The reasons for excluding trials will be recorded in the table ‘Characteristics of excluded studies’. We will include, and report characteristics of, studies in the review irrespective of whether measured outcome data are reported in a ‘usable’ way. Studies that meet the inclusion criteria will be included and described in the ‘Characteristics of included studies’ table, even if they do not report usable result. We will document the selection process in sufficient detail to complete a PRISMA flow chart12 and a table of ‘Characteristics of excluded studies’.
Data extraction and management
We will extract data from included studies and assess their risk of bias. Two review authors (CML and CM) will extract data from each included study independently and in duplicate using a tool developed for the review. We will resolve differences by discussion and, if necessary, arbitration by a third person. For each study with more than one control or comparison group for the intervention, we will extract the results for each intervention arm. We will not double count data within a meta-analysis and we will combine groups to create single pairwise comparisons as appropriate. For each study, we will record the following data:
-
Year of publication, country of origin and source of study funding
-
Details of the participants, including demographic characteristics and criteria for inclusion
-
Details of the study design
-
Comparisons and co-interventions
-
Details of the outcomes assessed, including method of assessment and adverse outcomes
-
Duration of follow-up and assessment of time points
Assessment of risk of bias in included studies
Two review authors (CML and CM) will independently assess risk of bias for each randomized controlled study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.13 Domains covered will include:
-
sequence generation;
-
allocation concealment;
-
blinding;
-
incomplete outcome data; and
-
selective outcome reporting.
We will judge each potential source of bias as ‘unclear’, ‘low risk’ or ‘high risk’, and provide justification in the ‘Risk of bias’ table. The methodological quality of included non-randomized studies will be assessed using the Newcastle-Ottawa scale14 for cohort studies. Domains covered will include selection, comparability and outcome.
Measures of treatment effect
For dichotomous outcomes (survival of prostheses at time X, tooth survival at time X, biological/technical complications), the odds ratio (and 95% confidence interval) will be extracted from each study, before and after adjustment for any baseline differences (using logistic regression). Time to failure of prostheses or remaining teeth will be compared between intervention and control, by calculating hazard ratios (and 95% confidence intervals). For continuous outcomes (OHRQoL), the difference in mean change (and 95% confidence intervals) will be extracted. These mean differences will be pooled across studies using weighted mean differences or standardized mean differences, if different measurement scales are used. A sensitivity analysis will be included to test the robustness of results by excluding the contribution of studies with an overall high/unclear risk of bias.
Unit of analysis issues
All included studies will be assessed to determine the unit of randomization or selection and whether this is consistent with the unit of analysis.
Dealing with missing data
To facilitate any meta-analysis, where possible treatment estimates based upon multiple imputation will be used.
Assessment of heterogeneity
We will assess the significance of any discrepancies in the estimates of the treatment effects from the different studies by means of Cochrane’s test for heterogeneity (χ2-test), where P<0.1 will be considered significant. We will use the I2 statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than chance, to quantify heterogeneity, where an I2 statistic over 50% may represent substantial heterogeneity.13
Assessment for reporting biases
If there are ⩾10 studies included in any meta-analysis, the potential for publication bias will be explored using funnel plots to evaluate asymmetry.13
Data synthesis
Expert statistical advice will be sought before undertaking any meta-analyses. If studies are sufficiently homogenous in terms of design and comparator, meta-analyses will be conducted using a fixed or random-effects model. If tests of heterogeneity are not significant, each outcome will be combined and calculated using the fixed effects model. If statistical heterogeneity is observed (I2>50% or P<0.1), the random effects model will be chosen. If heterogeneity is substantial, a meta-analysis will not be performed and a narrative, qualitative summary will be done.
Subgroup analysis and investigation of heterogeneity
Subgroup comparisons are by their nature observational and so subject to bias. If there are sufficient studies, we will aim to carry out subgroup analyses to assess clinical heterogeneity, on the following basis:
-
Patient characteristics (age, sex, number of teeth)
-
Intervention type
-
Setting (general dental services, hospital dental services, community dental services)
-
Study design
We will only undertake meta-analyses if there are studies of similar comparisons reporting the same outcome measures.
Discussion
We will use summary tables to present the findings for the main comparisons in the review, to interpret the results and draw conclusions about the effects of different interventions, including the size of the effects and certainty of evidence. The quality of evidence for all outcomes (head-to-head comparisons) will be judged using the GRADE system. Factors considered will include study design, bias, inconsistency and level of precision. Quality will be adjudicated as high (further research is unlikely to change our confidence in the estimate of effect), moderate, low or very low (very uncertain about the estimate of effect). This review will assess the body of evidence for clinical decision making in patients with SDA and reduced dentitions, by comparing the effectiveness of different tooth replacement strategies. Additionally, by including subjective qualitative outcomes, such as OHRQoL, it will assess the influence of patients in this decision making. With evidence of income-related barriers to oral healthcare for many older adults,10 the results will help to inform subsequent cost-effectiveness analyses. This review will also help to identify areas of further research and hopefully inform future healthcare policy.
References
United Nations Department of Economic and Social Affairs Population DivisionWorld Population Ageing 2013. United Nations: New York, NY, USA, 2013.
Watt RG, Steele JG, Treasure ET, White DA, Pitts NB, Murray JJ . Adult Dental Health Survey 2009: implications of findings for clinical practice and oral health policy. Br Dent J 2013; 214: 71–75.
Marcenes W, Kassebaum NJ, Bernabe E, Flaxman A, Naghavi M, Lopez A et al. Burden of oral conditions in 1990-2010: a systematic analysis. J Dent Res 2013; 92: 592–597.
Gotfredsen K, Walls AW . What dentition assures oral function? Clin Oral Implants Res 2007; 18 (Supp 3): 34–45.
Gerritsen AE, Allen PF, Witter DJ, Bronkhorst EM, Creugers NH . Tooth loss and oral health-related quality of life: a systematic review and meta-analysis. Health Qual Life Outcomes 2010; 8: 126.
Kayser AF . Shortened dental arches and oral function. J Oral Rehab 1981; 8: 457–462.
World Health Organisation. Recent advances in oral health. WHO Technical Report Series No. 826. WHO: Geneva, 1992: 16–17.
Kanno T, Carlsson GE . A review of the shortened dental arch concept focusing on the work by the Kayser/Nijmegen group. J Oral Rehab 2006; 33: 850–862.
Abt E, Carr AB, Worthington HV . Interventions for replacing missing teeth: partially absent dentition. Cochrane Database Syst Rev 2012, 2CD003814.
Listl S . Income-related inequalities in dental service utilization by europeans aged 50+. J Dent Res 2011; 90: 717–723.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 349: g7647.
Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535.
Higgins JPT, Green S (editor). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Online information available at www.cochrane-handbook.org.
Wells G, Shea B, O’Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2013, Online information available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Acknowledgements
The authors like to thank Richard Fallis (Subject Librarian, Queen’s University Belfast) for his collaboration in developing search strategies for this systematic review.
Author information
Authors and Affiliations
Contributions
CML is the principal investigator of this project and guarantor. CML drafted the manuscript, including development of selection criteria, risk of bias assessment strategy, data extraction criteria and search strategy. GMK and MD are co-investigators of this project and contributed to the writing and revision of the manuscript. All authors read, provided feedback and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
McLister, C., Donnelly, M., Cardwell, C. et al. Effectiveness of prosthodontic interventions and survival of remaining teeth in adult patients with shortened dental arches—Protocol for a systematic review and meta-analysis. BDJ Open 4, 17023 (2018). https://doi.org/10.1038/bdjopen.2017.23
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/bdjopen.2017.23