Abstract
We examined four clinically assessed cytogenetic subtypes (t(11;14), t(4;14), monosomy 13/del13q and monosomy 17/del17p in 292 black patients with newly diagnosed multiple myeloma (MM) from four medical centers, who had fluorescent in situ hybridization testing results available in their medical records. We then compared the prevalence of these abnormalities with a previously characterized Mayo Clinic cohort of 471 patients with MM. We found a significant difference in the prevalence of the t(11;14) immunoglobulin heavy chain (IgH) translocation between blacks and whites, 6.5% versus 17.6%, respectively, P<0.0001. Blacks also had lower rates of the t(4;14) IgH translocation, (5.5% versus 10%); monosomy 13/del13q (29.1 versus 49.3%); and monosomy 17/del17p (7.9% versus 13%). Consequently, 63.4% of blacks versus 34.6% of whites did not have any of the four abnormalities that we studied, P<0.001. As almost all MM is associated with either an IgH translocation or trisomies, we hypothesize that MM in blacks is associated with either excess prevalence of either the trisomic (hyperdiploid) form of MM or an IgH translocation besides t(11;14) or t(4;14). We conclude that there are significant differences in the cytogenetic subtypes of MM that occur in blacks and whites.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is a clonal plasma cell proliferative malignancy that is associated with a diverse set of clinical manifestations.1, 2, 3 It is also one of the malignancies with the greatest disparities in prevalence and incidence between blacks and whites.4, 5, 6 Several studies have reported between twofold and threefold increased risk of MM in blacks compared with whites.7, 8 This increased risk is thought to be the result of an increased prevalence of the premalignant condition, monoclonal gammopathy of undetermined significance in blacks.4 The increased risk of monoclonal gammopathy of undetermined significance in blacks compared with whites is seen in both African Americans as well as blacks from Ghana suggesting a genetic predisposition.9 The racial disparity in prevalence of monoclonal gammopathy of undetermined significance is present even after adjusting for socio-economic status.10 A recent Surveillance Epidemiology and End Results-based study found that black MM patients had significantly better disease-specific survival rates compared with whites (P<0.001).11
MM has several primary cytogenetic subtypes that can be broadly divided into two groups: translocations involving the immunoglobulin heavy chain (IgH) locus on 14q32, and trisomies of odd-numbered chromosomes (referred to as trisomic or hyperdiploid MM).12, 13 The most common IgH translocations include t(11;14), t(6;14), t(4;14), t(14;16) and t(14;20); the latter three are generally associated with more adverse prognosis. In addition to the primary cytogenetic abnormalities discussed above, there are additional abnormalities that have been associated with MM that can be seen either from the onset of the disease such as monosomy 13/del13q or mainly with disease progression such as del17p. Unlike primary cytogenetic abnormalities that are typically non-overlapping, these secondary changes are overlapping in the sense that they can be seen in combination with any of the primary cytogenetic subtypes.
A study utilizing samples from MM trials conducted by the Eastern Cooperative Oncology Group (ECOG) found that African American patients with MM have a lower frequency of IgH translocations compared with whites (40% versus 52%, P=0.032).14 As almost all patients with MM have IgH translocations, trisomies or both, this finding suggests that a higher proportion of MM in African Americans is either of the trisomic subtype of MM. However, more data are needed on the distribution of the major cytogenetic abnormalities by race in MM. In this study, we sought to determine whether the distribution of the four most common cytogenetic abnormalities, t(11;14), t(4;14), del13q and del17p, differs in blacks compared with whites in a multi-center national study using medical records and fluorescent in situ hybridization (FISH) studies conducted as part of routine clinical care.
Materials and methods
Participating institutions
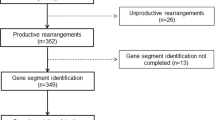
Collaborations were established between Mayo Clinic (Rochester, MN, USA) and three major institutions with large African American clinical practices: University of Maryland at Baltimore, MD, USA; Cook County Hospital, Chicago, IL, USA; and Rush University Medical Center, Chicago IL, USA. Data were abstracted by AJG from the records of 292 eligible black patients across the four participating institutions. Institutional Review Board’s approval was obtained from all sites.
Case eligibility and data collection
Patient data were collected according to a standardized case report form developed at Mayo Clinic. All patient data were de-identified and replaced with a study code. Inclusion criteria for African American patients were that (1) they were newly diagnosed (not relapsed), (2) diagnosed between 1 January 2006 and 31 January 2013 and (3) had clinical cytogenetic and FISH analyses available in the medical record within year before or 6 months after diagnosis. This yielded 292 eligible patients. Specifically, information regarding probes and clinical results for t(11;14), t(4;14), monosomy 13/del13q and del17p on FISH testing was abstracted. Patients were then categorized based on the presence/absence of these abnormalities. These abnormalities were chosen for this study, because probes to detect these are used almost universally, enabling comparisons between blacks and whites studied at various centers. In contrast, probes to detect trisomies and other IgH translocations are used less often, and racial differences may reflect probe strategy rather than true differences. We classified individuals without any of these four abnormalities as ‘potential other’ to indicate that they likely harbor other cytogenetic abnormalities, but reliable comparisons were not possible, because the probes to detect these were not used uniformly across the various centers. Data on African American patients were compared with an established sample of 471 white Mayo Clinic MM patients.5
Statistical analysis
Differences for each of the four cytogenetic abnormalities (t(11;14), t(4;14), monosomy 13/del13q and del17p) were compared individually between blacks and whites enrolled in the study using the Chi-squared tests. Cochran–Mantel–Haenszel tests were conducted to determine whether there were significant differences in cytogenetic abnormality by age and race.
Results
Of the patients eligible, data were available and collected on 292 African American MM patients from the four participating institutions and the comparison group of 471 white patients from Mayo Clinic (Table 1). Median age at diagnosis was significantly younger in blacks (59 years) compared with whites (63 years; P<0.0001). A significant difference was found in the distribution of gender between the two patient groups (44.5% male in blacks, 60.1% male in whites; P<0.0001).
When examining the overall distribution across the four different cytogenetic subtypes, we found evidence of a significant difference by race (Table 1, P<0.0001). Further analysis of the individual cytogenetic abnormalities demonstrated lower frequencies of each of the four abnormalities in blacks compared with whites (Table 1). There was a lower prevalence of the t(11;14) translocation by race in blacks compared with whites, 6.5% in blacks versus 17.6% in whites, P<0.0001. A difference was also observed in the prevalence of monosomy 13/del13q by race, 29.1 versus 47.3%, respectively, P<0.0001. Blacks also had lower rates of t(4;14) and monosomy 17/del17p compared with whites (Table 1). Also, 63.4% of blacks had none of the four abnormalities studied compared with 34.6% of whites, P<0.0001.
Patients were then stratified by age into <60 years or ⩾60 years (Table 2). Within each age group, blacks showed lower frequencies of the four abnormalities than whites. Conversely, whites in both age groups had lower frequencies of ‘potential other’ abnormalities (Table 2, P<0.0001). Black patients who were aged ⩾60 years had the lowest frequency of del17p (5.7%) compared with other age groups in both races.
Discussion
In the present study, we conducted a multicenter study to assess the frequency of four cytogenetic abnormalities routinely tested for with FISH probes in black patients with MM. We then compared these results with 471 white patients with MM seen at the Mayo Clinic in Rochester, MN, USA who were part of a well-defined cohort of MM that we have previously studied.2, 12 Although MM is clinically considered as a unique disease, it is likely a collection of several cytogenetically unique malignancies that are considered together as one entity solely, because they arise from plasma cells and have roughly similar clinical features.15 As the racial disparity in the incidence of MM is marked (relative risk of ⩾2 in blacks), it is unlikely that the increase in risk is shared by all cytogenetic subtypes of the disease. Our hypothesis is that the racial predisposition in MM is driven largely by an excess risk of one or more specific cytogenetic subtypes of MM.
In this study, we found that the frequency of the t(11;14) translocation is significantly lower in blacks compared with whites, and this difference was similar across age. We also found a lower rate of the t(4;14) translocation. Our findings are similar in this regard to that reported by Fonseca and colleagues.14 In our study, 63.4% of blacks versus 34.6% of whites did not have any of the four abnormalities that we studied, P<0.001. As almost all MM is associated with either an IgH translocation or trisomies (or both together), we hypothesize that MM in blacks is associated with either excess prevalence of either the trisomic (hyperdiploid) form of MM or an IgH translocation besides t(11;14) or t(4;14). Based on earlier studies by Fonseca and colleagues showing lower rate of IgH translocations in blacks,14 we hypothesize that most of the disparity is due to a higher prevalence of trisomic form of MM in blacks. This form of MM has a better prognosis12 and has a better outcome with lenalidomide therapy. Thus one would expect that blacks with MM, especially those treated with lenalidomide, will have a better outcome.16 In fact, in a recent ECOG randomized trial that utilized lenalidomide in both arms, although response rates were similar, overall survival was significantly superior in non-whites (almost all blacks) compared with whites, supporting this hypothesis.17 However, additional studies with information on trisomies and treatment will be important to confirm this hypothesis.
In addition, we found lower rates of monosomy 13/del13q and monosomy 17/del17p in blacks. Monosomy 17p/del17p has been associated with shorter survival in MM, and the disparity in prevalence of this cytogenetic abnormality may provide an additional explanation for the difference in survival seen between black and white MM patients. The effect of monosomy 13/del13q detected on FISH is of uncertain prognostic significance.
This is one of the largest studies comparing the prevalence of cytogenetic abnormalities in blacks versus whites with MM. It also draws strengths from being multi-centered and having a well-defined control group. However, as with any medical record-based investigation, there are limitations. First, as institutions and clinical laboratories use different probe-sets, we were limited in which cytogenetic abnormalities we could examine with confidence. Second, it is possible that differences in FISH methods across institutions, for example, whether there was sorting of CD138-positive cells or not, and the thresholds for positive and negative, may have affected our results. Third, we could not match patients by key clinical variables, although we tried to limit the effects of age as shown by the analysis in Table 2. Further study needs to be conducted using patients matched by race and geographic region and patients studied with uniform probe-sets to determine whether these biases are influencing our results. Finally, it is possible that there may be differences in the type of patients who are referred to specialized institutions that could affect the comparisons. Future studies should include a comprehensive MM probe panel so as to be able to determine the exact prevalence of additional translocations and trisomies across racial groups. These will be of greater importance as the treatment options available for the disease increase,18, 19 and it is likely that there will be variations in response to therapy based on the underlying cytogenetic subtype and race.
Change history
13 February 2015
This article has been corrected since Online Publication and an erratum has also been published
References
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol 2014; 15: e538–e548.
Greenberg AJ, Rajkumar SV, Therneau TM, Singh PP, Dispenzieri A, Kumar SK . Relationship between initial clinical presentation and the molecular cytogenetic classification of myeloma. Leukemia 2014; 28: 398–403.
Rajkumar SV . Multiple myeloma: 2014 Update on diagnosis, risk-stratification, and management. Am J Hematol 2014; 89: 998–1009.
Landgren O, Graubard BI, Katzmann JA, Kyle RA, Ahmadizadeh I, Clark R et al. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12 482 persons from the national health and nutritional examination survey. Leukemia 2014; 28: 1537–1542.
Landgren O, Gridley G, Turesson I, Caporaso NE, Goldin LR, Baris D et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood 2006; 107: 904–906.
Greenberg AJ, Vachon CM, Rajkumar SV . Disparities in the prevalence, pathogenesis and progression of monoclonal gammopathy of undetermined significance and multiple myeloma between blacks and whites. Leukemia 2012; 26: 609–614.
Brown LM, Linet MS, Greenberg RS, Silverman DT, Hayes RB, Swanson GM et al. Multiple myeloma and family history of cancer among blacks and whites in the U.S. Cancer 1999; 85: 2385–2390.
Cohen HJ, Crawford J, Rao MK, Pieper CF, Currie MS . Racial differences in the prevalence of monoclonal gammopathy in a community-based sample of the elderly.[erratum appears in Am J Med 1998;105(4):362]. Am J Med 1998; 104: 439–444.
Landgren O, Katzmann JA, Hsing AW, Pfeiffer RM, Kyle RA, Yeboah ED et al. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin Proc 2007; 82: 1468–1473.
Landgren O, Rajkumar SV, Pfeiffer RM, Kyle RA, Katzmann JA, Dispenzieri A et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance (MGUS) among African-American and Caucasian women. Blood 2010; 116: 1056–1059.
Waxman AJ, Mink PJ, Devesa SS, Anderson WF, Weiss BM, Kristinsson SY et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood 2010; 116: 5501–5506.
Kumar S, Fonseca R, Ketterling RP, Dispenzieri A, Lacy MQ, Gertz MA et al. Trisomies in multiple myeloma: impact on survival in patients with high-risk cytogenetics. Blood 2012; 119: 2100–2105.
Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia 2009; 23: 2210–2221.
Baker A, Braggio E, Jacobus S, Jung S, Larson D, Therneau T et al. Uncovering the biology of multiple myeloma among African Americans: a comprehensive genomics approach. Blood 2013; 121: 3147–3152.
Fonseca R . Many and multiple myeloma(s). Leukemia 2003; 17: 1943–1944.
Pandey S, Rajkumar SV, Kapoor P, Ketterling RP, Lacy MQ, Gertz MA et al. Impact of FISH abnormalities on response to lenalidomide in patients with multiple myeloma. Blood 2013; 122: 3210.
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 2010; 11: 29–37.
van der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW et al. The therapeutic human CD38 antibody daratumumab improves the anti-myeloma effect of newly emerging multi-drug therapies. Blood Cancer J 2011; 1: e41.
Chanan-Khan AA, Swaika A, Paulus A, Kumar SK, Mikhael JR, Rajkumar SV et al. Pomalidomide: the new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J 2013; 3: e143.
Acknowledgements
This work is supported in part by grants CA 107476 and CA 168762 from the National Cancer Institute, Rockville, MD, USA. Also supported in part by the Mayo Clinic Hematological Malignancies Program; and the Henry J. Predolin Foundation, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
SVR and AJG conceived, designed the study, conducted all analyses and wrote the manuscript, with input from all authors; AJG abstracted all data; SVR, SK, RAK, RK, SP, AP, SV, AB and RC contributed data and identified patients for study from the participating institutions. All authors reviewed and approved the paper.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Greenberg, A., Philip, S., Paner, A. et al. Racial differences in primary cytogenetic abnormalities in multiple myeloma: a multi-center study. Blood Cancer Journal 5, e271 (2015). https://doi.org/10.1038/bcj.2014.91
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2014.91
This article is cited by
-
Racial differences as predictors of outcomes in young patients with multiple myeloma
Blood Cancer Journal (2022)
-
Dissecting racial disparities in multiple myeloma
Blood Cancer Journal (2020)
-
Racial differences in treatment and outcomes in multiple myeloma: a multiple myeloma research foundation analysis
Blood Cancer Journal (2020)
-
Biological determinants of health disparities in multiple myeloma
Blood Cancer Journal (2018)
-
Plasma Cell Dyscrasias in India-2017 Updates
Indian Journal of Hematology and Blood Transfusion (2018)