Abstract
WNT signaling has been implicated in the regulation of hematopoietic stem cells and plays an important role during T-cell development in thymus. Here we investigated WNT pathway activation in childhood T-cell acute lymphoblastic leukemia (T-ALL) patients. To evaluate the potential role of WNT signaling in T-cell leukomogenesis, we performed expression analysis of key components of WNT pathway. More than 85% of the childhood T-ALL patients showed upregulated β-catenin expression at the protein level compared with normal human thymocytes. The impact of this upregulation was reflected in high expression of known target genes (AXIN2, c-MYC, TCF1 and LEF). Especially AXIN2, the universal target gene of WNT pathway, was upregulated at both mRNA and protein levels in ∼40% of the patients. When β-CATENIN gene was silenced by small interfering RNA, the cancer cells showed higher rates of apoptosis. These results demonstrate that abnormal WNT signaling activation occurs in a significant fraction of human T-ALL cases independent of known T-ALL risk factors. We conclude that deregulated WNT signaling is a novel oncogenic event in childhood T-ALL.
Similar content being viewed by others
Introduction
It is generally accepted that T-cell acute lymphoblastic leukemia (T-ALL) results from malignant transformation of normal developing T cells in the thymus, the so-called thymocytes. T-ALL represents 15% of childhood and 25% of adult ALL. Chromosomal aberrations leading to abnormal fusion proteins, as often found as causative factor in precursor B-ALL, are not commonly found in T-ALL. However, aberrant regulations of signaling pathways that control normal T-cell development in the thymus are important for T-ALL leukomogenesis. These pathways are strictly regulated, as many of the key molecules in these pathways are considered proto-oncogenes. These oncogenes can be activated by rearrangement to one of the T-cell receptor loci that is, in fact, the case in at least one-third of T-ALL patients.1 Deregulated signaling is considered a major contributing factor in leukomogenesis of T-ALL. This is exemplified by the Notch pathway that is critically important for normal T-cell development but, when constitutively activated through somatic mutations, it invariably leads to T-ALL.2 Another pathway that is important for T-cell development and is similarly evolutionary conserved from flies and worms to mice and humans is the WNT pathway.3 WNT proteins are intercellular signaling molecules that regulate developmental processes such as cell-fate decisions, proliferation of progenitor cells and establishment of dorsal–ventral axis and control of asymmetric cell division.4 β-Catenin (β-catenin, CTNNB1) is the key mediator of canonical WNT pathway. In the presence of WNT proteins, β-catenin accumulates in the cytoplasm and moves to the nucleus where it interacts with transcription factors of the T-cell factor (Tcf)/lymphoid enhancer factor family. The complex of β-catenin/Tcf or β-catenin/Lef leads to target gene expression modulation in nucleus5 and cooperates in neoplastic transformation.6 The expression of WNT proteins in bone marrow indicates that they may influence fetal and adult development of hematopoietic stem cells.7 Lymphocyte progenitor cells appear to be regulated by WNT signals with respect to survival and expansion that may be partly mediated via the high specificity of immature progenitor cells toward WNT proteins.8, 9 Previous studies have shown that deregulation of the WNT pathway has potent oncogenic effects in tissues such as colon, breast and prostate.10, 11, 12 According to the previous studies, genetic or epigenetic deregulation of WNT signaling is also involved in pathogenesis of different hematological malignancies including chronic myeloid leukemia, chronic lymphoblastic leukemia, acute myeloid leukemia and ALL.13, 14 Of importance are mouse models showing that activated β-catenin can lead to T-cell lymphomas in the thymus14 and that loss of Tcf as tumor suppressor gene leads to Lef-1-dependent high Wnt signaling, causing aggressive T-cell lymphomas.15 However, many of these studies on hematological malignancies were performed in mouse models, showing the potential of aberrant Wnt signaling in T-All development, but revealed limited data about the role of WNT pathway in human T-ALL samples. In a previous study, we determined several variations in the APC and AXIN1 genes that may play a role in the regulation of the β-catenin protein levels in childhood ALL patients.16
In this study, we performed a more genome-wide analysis on the role of WNT signaling in childhood T-ALL, also focusing on several key components of this pathway. Consequently, we define abnormal WNT pathway activation in a group of childhood T-ALL cases, independent of Notch activation and other known chromosomal aberrations.
Materials and methods
Case–control selection
A total of 71 (18 girls and 53 boys) childhood T-ALL patients diagnosed at Istanbul Medical Faculty and Cerrahpasa Medical Faculty of Istanbul University were included in this study. Median age was 8.05 years (min–max 1.3–16.9) and median white blood cell count was 61 200 × 109/l (min–max 1000–603 000). The patients were diagnosed according to FAB (French–American–British) classification criteria and treated with the BFM (Berlin–Frankfurt–Munster)-ALL protocol. The bone marrow samples that were taken for diagnostic purposes, with a blast load of ⩾90% and the CD7 positivity of ⩾90%, were included in the study.17 The NOTCH1 and FBXW7 mutation rates of the study population were 21% and 10%, respectively.18 To be able to compare the patients with their stage-specific controls, the patients were classified according to the EGIL (European Group for the Immunological Characterization of Leukemias) proposal for T-ALL.19
We used thymocyte subsets (double negative (DN), immature single positive, double positive (DP) DP CD3+ and DP CD3−, single positive (SP) SP CD4+, SP CD8+, n=6) as controls that is defined by Weerkamp et al.8 The ethical committee of Istanbul Medical Faculty (reference number and date: 2008/305 and 20.02.2008) approved this study and written informed parental consents were obtained.
RNA isolation and cDNA synthesis
Total RNA was isolated by Qiagen RNeasy Plus Mini Kit (Qiagen GmbH, Hilden, Germany) and complementary DNA (cDNA) was synthesized by random hexamers and Moloney Murine Leukemia Virus reverse transcriptase from 1 μg of total RNA according to the manufacturer procedures (MBI Fermentas, Vilnius, Lithuania).
Microarray analysis
In all, 31 T-ALL samples were studied by microarray analysis. The RNA quality and quantity were detected by Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA) and Nanodrop ND1000 (Thermo Fisher Scientific, Lafayette, CO, USA). Affymetrix One cycle cDNA synthesis kit (Affymetrix, Santa Clara, CA, USA) was used for cDNA and cRNA synthesis. The samples were biotin labeled by Affymetrix IVT labeling kit. The samples were hybridized overnight at 42 °C with GeneChip HU-133 Plus.2 microarray chips and scanned by GeneChip scanner 3000 at the Department of Immunology, Erasmus Medical Center, Rotterdam, The Netherlands. Microarray data are available at http://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE46170).
Data analysis
Raw microarray data were normalized by the robust multiarray average method as described by Irizarry et al.20 Following normalization, the analysis was performed using BRB Array tools version 3.7.1 developed by Richard Simon and Amy Peng Lam.21 Microarray analysis was performed taking into account the guidelines as formulated for such studies by three large European consortia.17 As the tumor load was >90% in all cases, no purification was performed as indicated by the study by de Ridder et al.22 The normalized intensities applied to BRB array tools data filtering. The data that showed <20% expression values and a twofold change in either direction from the gene median value were excluded.
Analysis of gene expression by real-time quantitative PCR
Real-time quantitative PCR was carried out on the Light Cycler 480 Instrument (Roche Applied Sciences, Manheim, Germany). The specific primer probe sets are given at Supplementary Table 1. Real-time amplification was performed with Light Cycler 480 Probe Master Mix (Roche) as described in the protocol. For normalization, the most stable three genes (β-ACTIN, CYCLOPHILIN and ABL) were selected by GeNorm (V3.4, Amel, Belgium) software.23 Relative expressions were calculated according to the ΔΔCt method, based on the mathematical model described by Livak et al.24
Protein detection assays
For western blot analysis, lysates containing 20 μg of protein were separated by electrophoresis on a 10% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes. To detect total β-catenin (Catenin-β AB-2 rabbit polyclonal; Millipore, Billerica, MA, USA) and active-β-catenin (anti-ABC, clone 8E7, mouse monoclonal IgG1K; Millipore), two different antibodies were used. HEK293 cell line, both LiCl treated and not treated, was used as positive controls. For immunofluorescence assay, 1 × 106 cells were fixed by cytospin (Thermo Scientific, Waltham, MA, USA). In each staining, nuclei were detected by 4,6-diamidino-2-phenylindole and the analyses performed on digital florescence microscope (Leica CTR 6000, Leica, Wetzlar, Germany). We utilized the in situ proximity ligation assay (PLA) for detection of Axin 2 expression, and β-catenin/Tcf 4 protein interaction in patients and T-ALL cell lines.25 This method allows detection of proteins, protein–protein interactions using proximity probes (that is, antibodies conjugated to a short DNA strand) to target the individual partners involved in protein complex. β-Catenin was recognized by a rabbit monoclonal antibody (E247 to β-catenin; Abcam, Cambridge, MA, USA), Tcf4 was detected by goat polyclonal antibody (Tcf-4 C-19, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and we used a rabbit polyclonal to Axin2 (Axin2, Abcam). To detect the primary antibodies with in situ PLA, secondary proximity probes binding rabbit and goat immunoglobulin (PLA probe rabbit PLUS and MINUS, and PLA probe goat MINUS; Olink Bioscience, Uppsala, Sweden) were used respectively. The detection of the bound proximity probes was performed with in situ PLA detection kit 613 (Olink Bioscience) according to the manufacturer’s instructions.
Mutation analysis
T-ALL patients and cell lines were analyzed for the mutations of β-CATENIN (exons 2 and 3). The primers were used as follows; forward 5′-TTCCCCTGAGGGTATTTGAAGTAT-3′ and reverse 5′-CATGCCCTCATCTAATGTCTCAG-3′. Amplified samples were run with denaturing high-performance liquid chromatography (Transgenomic, Omaha, NE, USA). Different chromatograms were directly sequenced and analyzed by the CLC combined Workbench software (V.3.6.1, Aarhus, Denmark).
TOP/FOP Luciferase reporter assay
In order to detect the effect of the Q68P mutation, which was described first time by this study, on β-CATENIN expression, we designed a TOP/FOP luciferase assay.26 First, we purchased two β-CATENIN expression plasmids, one carrying the wild-type (Addgene plasmid 16828, Cambridge, MA, USA) and the other carrying the S33Y (Addgene plasmid 19286) mutation.27 Moreover, we have synthesized the Q68P mutation commercially (Gene Art, Invitrogen Life Sciences, Carlsbad, CA, USA). Afterwards, the HEK293 cells (300 000/well) were transfected by Fugene6 transfection reagent (Promega, Madison, WI, USA) as described by the manufacturer’s protocol with three different conditions. Dual-Glo Luciferase Assay kit is used (Promega) and luciferase expressions were measured on luminometry (Synergy MX, Biotech, Winooski, VT, USA). Each condition was studied in duplicate and the experiment was repeated for three different times.
Cell culture and siRNA treatment
Molt4 T-ALL cell line was kindly provided by Dr Anton W Langerak, Department of Immunology, Erasmus Medical Center. To repress β-CATENIN, a pool composed of four predesigned small interfering RNA (siRNA) (ON-TARGETplus SMARTpool Human-CTNNB1; Thermo Fisher Scientific) was used. Cells treated with nontargeting siRNA pool (Dharmacon, Inc., Lafayette, CO, USA), with only transfection reagent (mock) and untreated cells were used as controls. The siRNAs were transfected into Molt4 cells with Oligofect-AMINE reagent (Invitrogen). Cells were seeded into 24-well plates with a number of 3 × 105 viable cells/well, incubated for 12 h prior transfection, transfected with 20 μM siRNA and incubated for the indicated time points (24, 33, 48 and 72 h). The experiment was repeated for three different times.
Detection of apoptosis by flow cytometry
To analyze the percentage of apoptotic cells by flow cytometry, β-CATENIN siRNA-treated Molt4 cells were stained with FITC conjugated annexin V and propidium iodide (Apoptest-FITC; Dako, Hoeven, The Netherlands). Data were analyzed using the FACSDiva software application (Becton Dickinson, Pharmigen, San Jose, CA, USA).
Statistical analysis
Relative expressions were compared by two tailed t-test where appropriate. The P-value of <0.05 was considered statistically significant. The Kaplan–Meier method was used to estimate survival rates. The median follow up was 54.28 months (min–max 0.1–180 months). Overall survival was defined by the interval from the date of diagnosis to the date of death or last follow-up. Relapse-free survival was the duration from the date of complete remission to the date of analysis or to the first event (failure to achieve remission (early death or resistant leukemia), relapse or death in complete remission). Differences were compared with the two-sided log-rank test. All statistical analyses were done by SPSS for windows 19.0 (IBM SPSS Data Editor Inc., Chicago, IL, USA) and GraphPad Prism 5.04 software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Microarray study
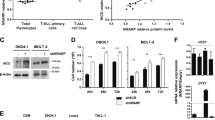
We defined a gene list comprising members of WNT signaling pathway based on the information obtained from public databases and web portals (NCBI Entrez Gene, The WNT Homepage (http://www.stanford.edu/~rnusse/wntwindow.html) to mine the microarray data for genes from the WNT pathway on 31T-ALL patients. The data set was filtered to exclude genes showing minimal variation across the set of arrays from the analysis. Following filtering, 12 WNT signaling member probe sets (TCF4 (also known as TCF7L2), CTNNB1, LRP6, LEF1, TCF1 (also known as TCF7), c-MYC and FZ6) were found to be differentially expressed with a P-value of <0.05 (Figure 1). A two-way clustering algorithm, namely complete linkage with ‘Euclidian distance’ correlation, was used to cluster genes and samples. Patients and control subsets were distributed differentially in the dendogram. Also, ∼50% of T-ALL patients showed at least twofold change in β-CATENIN expression. The gene for TCF4 gene was highly upregulated in the patient group, whereas TCF1 did not show differential expression in patients compared with controls. The heat map result shows that the WNT members are differentially expressed in a case-restricted manner and that high WNT signaling is not a feature common to all patients but restricted to a substantial subset of patients.
The heatmap diagram of WNT signaling members in T-ALL patients and controls. Gene and samples clustered using Euclidean distance and complete linkage method for the probe sets in 31 T-ALL patients, total thymus tissue and thymocyte subsets (SP4 (CD4 single positive), SP8 (CD8 single positive), DP total (CD4+CD8+ double positive CD3+/CD3), thymus (total thymus tissue), DP3− (CD4+CD8+ double positive CD3 negative), ISP (immature single positive) and DN (CD3−, CD4−, CD8−). The patient samples were clustered between the numbers 1 and 27 and coded with the letter T. Gene names are shown as they are represented by the annotation file of Affymetrix: CTNNB1, β-CATENIN, TCF7L2(TCF4), LRP6, LEF1, TCF7 (TCF1), MYC and FZD6.
Elevated levels of β-catenin in childhood T-ALL
To validate the array findings, we studied in a patient group (n=71) the expression levels of the key molecule β-CATENIN by quantitative real-time PCR. The mRNA expression (P=0.007, Figure 2a) and protein expressions (Figure 2c) were found to be significantly upregulated in the majority of the patients. To detect whether this accumulation leads to translocation of active β-catenin into the nucleus, we also performed immunofluorescence staining in primary patient samples and showed activated (dephosphorylated) β-catenin in the nucleus of T-ALL cases (Figure 2b).28
Elevated levels of β-CATENIN in T-ALL patients. (a) Elevated expression of β-CATENIN in T-ALL samples compared with thymic subsets (SP4+, SP8+, DP3+, DP total and total thymocytes). The horizontal bar represents mean value. mRNA levels are normalized to the geometric mean of three reference genes (ABL, β-ACTIN and CYPA). The gene expression level of β-CATENIN was significantly higher in T-ALL patients as compared with normal T cells (P=0.007, by Mann–Whitney test). (b) Immunofloresence analysis of activated Molt4 cell lines and two representative T-ALL patients (T103 and T136 are patient numbers) were immunostained for β-catenin (red) and active-β-catenin (green). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI, blue). Original magnification × 100. Active-β-catenin (dephosphorylated β-catenin) immigrates into the nucleus. (c) Western blot analysis of total and active β-catenin in T-ALL patients and total thymocytes. Results of six representative patients are shown (T9, T54, T71, T83, T53 and T81 are patient numbers). Protein level was normalized to β-actin (bottom panel).
To identify whether the observed active β-catenin-mediated WNT signaling in T-ALL patients is caused by mutations in the β-CATENIN gene, the hot spot regions were analyzed using denaturing high-performance liquid chromatography and direct sequencing. Only in one patient, a novel variation for β-CATENIN (c.418A>C, P.Q68P, Figure 3a) has been identified. TOP/FOP reporter assay analysis revealed that Q68P mutated plasmid had significantly elevated β-catenin activity when compared with wild-type and S33Y mutant β-CATENIN (P=0.004, Mann–Whitney U test; Figure 3b).
β-CATENIN mutation was detected in one patient. (a) The sequence analysis was done by CLC work bench. The forward and reverse reads are aligned to the reference sequence, and reverse read is taken as reverse complement. Direct sequencing diagram c.418 A>C, P.Q68P. (b) TOP/FOP reporter assay analysis revealed that Q68P mutated plasmid had significantly elevated β-catenin activity when compared with wild-type and S33Y mutant β-CATENIN. Luciferase reads from TOP/FOP reporter assay. Q68P, Q68P mutated β-catenin plasmid; S33Y, S33Y mutated β-catenin plasmid; WT, wild type β-catenin plasmid. 3 × 105 HEK293 cells/well were transfected by Fugene6 transfection reagent. All three β-CATENIN plasmids were co-transfected with TOP and FOP plasmids. Each well was also transfected with a plasmid expressing Renilla luciferase as an internal control.
Defining the interaction of β-CATENIN and TCF/LEF transcription factors
At first, the mRNA expressions of TCF/LEF transcription factors were studied in T-ALL patients, and TCF4 (TCF7L2, P=0.004, Figure 4a) and LEF1 (P=0.002, Figure 4c) genes showed high levels of mRNA expression, whereas no significant difference was found for TCF1 (TCF7, P=0.13, Figure 4b). Then, we showed the protein–protein interactions between activated β-catenin and TCF/LEF transcription factors in the primary patient samples (Figure 4d) and cell lines, Molt4 and CEM (Figures 4e and f respectively) by proximity ligation assay.
Expression of transcription factors Tcf/Lef and their interactions with β-Catenin. (a) TCF4, (b) TCF1 and (c) LEF1 relative expression levels were detected by real-time quantitative PCR (RQ-PCR) in T-ALL patients. Patients were compared with their representative thymic subsets. mRNA levels are relative to the geometric mean of three reference genes (ABL β-ACTIN and CYPA). Colocalization of β-catenin/Tcf4 was detected by in situ PLA in (d) one T-ALL patient, (e) Molt4 cell line and (f) CEM cell line. Red indicates in situ PLA signals, and blue indicates cell nuclei.
Effects on downstream genes
Downstream target genes of β-catenin such as AXIN2, CCNDI and c-MYC were studied, to define the impact of β-catenin and TCF/LEF interaction. CCND1 showed a significant upregulation (P<0.001), whereas c-MYC expression did not show any difference between controls and samples (P=0.26; Supplementary Figure 1). To evaluate the active WNT signaling among childhood T-ALL patients, we mainly focused on AXIN2 gene, as it is considered a universal WNT target. The mRNA of AXIN2 gene was high in a significant number of cases (28 out of 71) and almost half of these patients were at the immature stage (TI/TII vs DN controls P=0.001, Figure 5a). Axin2 protein was also detected by in situ PLA (Figures 5c–e) and by western blot (Figure 5f) in T-ALL patients.
AXIN2 expression in primary patient samples and cell lines. (a) Elevated mRNA levels of AXIN2 in T-ALL samples compared with their representative thymic subsets (DN, double negative; DP, double positive; SP, single positive). (b–e) In situ PLA signals demonstrating expression of Axin2; Jurkat cell line was used as a positive control (b), three different T-ALL patients were represented in (c–e). Red indicates in situ PLA signals for Axin2, blue indicates cell nuclei. Original magnification × 100. (f) Axin2 expression was also verified by western blot analysis, and CEM and Jurkat cell lines were used as positive controls.
Inhibited β-CATENIN expression leads T cells to apoptosis
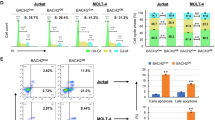
To address the results of upregulated WNT signaling we designed a siRNA experiment by using a siRNA cocktail which consists of four different siRNA to block β-CATENIN gene in the T-ALL cell line Molt4. We determined apoptotic effects of decreased β-CATENIN expression. The β-catenin-targeted siRNA treatment suppressed the β-CATENIN expression threefold on mRNA level at 24 h and the expression remained the same at 33 and 48 h, whereas the effect was lower at 72 h (Figure 6a). This suppression was also confirmed in protein level by in situ PLA (Supplementary Figure 2). We assessed the effects of β-CATENIN suppression on apoptosis at 48 h and detected increase in the apoptosis. We analyzed the three independent experiments, and β-CATENIN siRNA-treated cells showed significantly high apoptosis rate according to the controls (P=0.003) (Figures 6b and c).
β-CATENIN siRNA treatment promotes apoptosis in leukemic T cells. The cells were cultured in RPMI-1640 (Invitrogen Life Technologies) supplemented with 10% FCS, penicillin (50 U/ml) and streptomycin (50 mg/ml; both from Invitrogen Life Sciences). Molt4 cell line was transfected with 100 nM of anti-β-catenin siRNA and nontargeting control siRNA. Reduction of β-CATENIN mRNA expression was determined at 24, 33, 48 and 72 h after transfection. (a) β-CATENIN siRNA-treated samples (B24-72) compared with untreated cells (U24-72). (b) Determination of percentage of apoptotic cells by staining Annexin V and propidium iodide (PI) after 48 h of treatment. Apoptotic cells defined as AnnV+ PI+ cells. Representative plots from one experiment is shown. (c) Bar plots indicate the mean value of three independent apoptosis experiments. Apoptosis rate was significantly higher (P=0.003) in β-CATENIN siRNA-treated cells as compared with controls.
Defining WNT-active patient group
Here we showed a group of T-ALL patients with high AXIN2 levels (∼40%), an indication of active WNT signaling. To elucidate the cause of ectopically high WNT signaling in our cohort, we performed a supervised clustering analysis between patients with high and normal AXIN2 mRNA levels. The most differentially expressed genes are shown in Supplementary Table 2. The list contains genes that play a role in T-cell and hematopoietic development, with stem cell and non-T-cell lineage genes highly expressed in the AXIN2-high group, whereas T cell-specific genes associated with T-cell commitment were found in the AXIN2-normal group.
When the clinical features of the patients with activated WNT signaling were analyzed, according to their maturation steps 41% of these cases were falling in TI/TII group, whereas only 29% of the patients with normal AXIN2 levels were classified as TI/TII. Sex (P=0.14), white blood cell counts (P=0.51), risk group (P=0.12), central nervous system involvement (P=0.78) and high AXIN2 levels showed no significant correlation, whereas other organ involvements were detected more frequently in the WNT-active group (P<0.001). The Kaplan–Meier estimate of probability of both overall (P=0.16) and relapse-free survival (P=0.34) according to AXIN2 expression levels showed no significant difference, but the survival rates of patients with high AXIN2 levels were lower than the patients with normal AXIN2 levels (Figures 7a and b). We also checked whether high AXIN2 levels are independent from NOTCH1 mutations and found no significant difference between the NOTCH1 mutation rate and AXIN2-high (P=0.53) and AXIN2-normal (P=0.55) patients. The AXIN2 expression levels were also compared with the expression levels of known T-ALL oncogenes (LMO2, LYL1, TLX1, TLX3, BMI and TAL1) and no correlation was found.
Discussion
In the present study, we aimed to clarify the involvement of WNT pathway in the pathogenesis of human T-ALL and showed a significant subset of T-ALL patients displaying β-catenin-mediated WNT signaling that may alter cell characteristics. Although Wnt signaling is not thought to involve high mRNA levels of β-CATENIN, high (nuclear) protein expression of β-catenin is a typical characteristic of Wnt signaling. Here we found that in most patients high mRNA expression correlated with high protein expression, and using an antibody specific for the active form of β-catenin, high, nuclear β-catenin was found, indicative of active Wnt signaling in these leukemic cells. More than 85% of the childhood T-ALL patients showed upregulated β-CATENIN expressions. To identify whether this activation is ligand independent, T-ALL patients were screened for β-CATENIN mutations and a novel nonsynonymous alteration (Q68P) was detected in one patient. TOP/FOP assay showed increased β-CATENIN activity when compared with wild-type and S33Y mutant, and when we checked this individual patient, both microarray and mRNA expressions were extremely higher than in controls. Although this is a novel variation, another mutation was reported in the same codon previously (Q68*stop) in a gastric carcinoma patient.29 Recently, Groen et al.13 published known β-CATENIN mutations in T-lineage lymphomas, giving us the idea that β-CATENIN mutations take part in T-cell differentiation abnormalities. We previously determined the mutations in β-catenin degradation complex genes; APC and AXIN1 in childhood acute leukemia patients.16 Unlike solid tumors,30, 31 the mutation frequencies in AXIN1, APC and CTNNB1 genes together accounted for only ∼5% of the T-ALL patients, and this gives the idea that the contribution of these mutations far exceeded T-cell leukemogenesis. All these findings may imply that high levels of β-catenin give a survival advantage to malignant T cells, and according to our findings when the expression was suppressed by siRNA, cells are led to apoptosis in vitro.
This study also showed the interaction of β-catenin and Tcf/Lef transcription factors in primary patient samples that are previously known to regulate the cells to differentiate, grow or divide.32, 33 Both Tcf/Lef transcription factors are expressed in developing T lymphocytes in the thymus34, 35 and required for normal thymocyte survival and differentiation.8, 36 These transcription factors interact with both co-activator and corepressor proteins and, depending on the β-catenin existence, they activate the downstream targets.37 TCF4 overexpression was shown in some solid tumors38 but there are no available data on acute leukemia development. On the other hand, there are controversial findings regarding overexpression of LEF1 that was correlated with bad prognosis in adult pre-B ALL, whereas it is considered a good prognosis marker in cytogenetically normal acute myeloid leukemia.39, 40 Gutierrez et al.41 showed loss of Lef1 activity caused by microdeletions and truncating mutations especially in cortical stage T-ALL patients. It should be noted that LEF1 is also a WNT target gene that can not only mediate WNT signaling but also repress it, depending on the isoform expressed. In our study, high LEF1 merely indicates high WNT signaling. All the studies mentioned above including our findings are supporting the two-sided action of LEF1 gene.
Downstream targets of WNT signaling, CCND1 and AXIN2, both showed increased expressions in our patient cohort. TCF4, which is also found to be upregulated here, is one of the transcriptional regulators of CCND1 gene42 and this upregulation can be explained as due to β-catenin-mediated WNT signaling. As CCND1 is activated through several mechanisms, it is not a specific indicator of WNT activation. On the other hand, Axin2 expression is restricted to the regions that have increased β-catenin expression and is considered a universal WNT target gene.43 In this study, along with increased levels of β-catenin, a group of patients had high Axin2 expressions, which we define as WNT-active group, and, interestingly, a majority of these patients were classified in TI/TII, the so-called immature stage. An intriguing possibility is that the deregulated WNT signaling in the AXIN2-high (WNT-active) group may impose an immature phenotype on the T-ALL cells and even confer self-renewal properties on these cells or a subpopulation thereof (leukemic stem cells). There was no significant effect of high AXIN2 expression on survival rates, whereas organ involvement was significantly higher in this group of patients that refers to more aggressive forms of the disease.
Findings discussed above indicate abnormal WNT activation in a significant group of T-ALL patients. To identify whether this activation is related to other known genes in T-ALL pathogenesis (such as NOTCH1 mutations or T-ALL oncogene expressions like LMO2, LYL, TAL1 and so on) we evaluated these risk factors for our patient group in which we previously showed that NOTCH1 gene mutation frequency was 22%.18 NOTCH1 mutation frequencies in WNT-active patient group showed no significant difference when compared with the nonactive group. Moreover, ectopic expressions of T-ALL-specific oncogenic transcription factors (LMO2, LYL1, TLX1, TLX3, BMI and TAL1) in the same patient group44 showed no specific accumulation in either the active or inactive state of WNT. Although our findings show an active WNT signaling in childhood T-ALL, other mechanisms may operate as well, for instance as shown in mammary carcinoma, where excessive autocrine or paracrine WNT production causes high WNT signaling in tumor cells.45
Findings reported here support the notion that a subset of T-ALL cases, independent from the known risk factors discussed above, is characterized by deregulated WNT signaling. Because several small-molecule inhibitors exist that target Wnt signaling, these findings of abnormally high Wnt signaling in T-ALL offer the possibility of new therapeutic strategies.
Accession codes
References
Staal FJ, Langerak AW . Signaling pathways involved in the development of T-cell acute lymphoblastic leukemia. Haematologica 2008; 93: 493–497.
Weng AP, Lau A . Notch signaling in T-cell acute lymphoblastic leukemia. Future Oncol 2005; 1: 511–519.
Chien AJ, Conrad WH, Moon RT . A Wnt survival guide: from flies to human disease. J Invest Dermatol 2009; 129: 1614–1627.
Staal FJ, Clevers HC . WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol 2005; 5: 21–30.
Gordon MD, Nusse R . Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 2006; 281: 22429–22433.
Behrens J, Lustig B . The Wnt connection to tumorigenesis. Int J Dev Biol 2004; 48: 477–487.
Reya T, Clevers H . Wnt signalling in stem cells and cancer. Nature 2005; 434: 843–850.
Weerkamp F, Baert MR, Naber BA, Koster EE, de Haas EF, Atkuri KR et al. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci USA 2006; 103: 3322–3326.
Staal FJ, Weerkamp F, Baert MR, van den Burg CM, van Noort M, de Haas EF et al. Wnt target genes identified by DNA microarrays in immature CD34+ thymocytes regulate proliferation and cell adhesion. J Immunol 2004; 172: 1099–1108.
Dejmek J, Dejmek A, Safholm A, Sjolander A, Andersson T . Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res 2005; 65: 9142–9146.
Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci USA 2006; 103: 5454–5459.
Yang X, Chen MW, Terry S, Vacherot F, Bemis DL, Capodice J et al. Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene 2006; 25: 4256.
Groen RW, Oud ME, Schilder-Tol EJ, Overdijk MB, ten Berge D, Nusse R et al. Illegitimate WNT pathway activation by beta-catenin mutation or autocrine stimulation in T-cell malignancies. Cancer Res 2008; 68: 6969–6977.
Guo Z, Dose M, Kovalovsky D, Chang R, O'Neil J, Look AT et al. Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood 2007; 109: 5463–5472.
Tiemessen MM, Baert MR, Schonewille T, Brugman MH, Famili F, Salvatori DC et al. The nuclear effector of Wnt-signaling, Tcf1, functions as a T-cell-specific tumor suppressor for development of lymphomas. PLoS Biol 2012; 10: e1001430.
Erbilgin Y, Ng OH, Mavi N, Ozbek U, Sayitoglu M . Genetic alterations in members of the Wnt pathway in acute leukemia. Leuk Lymphoma 2011; 53: 508–510.
Staal FJ, Cario G, Cazzaniga G, Haferlach T, Heuser M, Hofmann WK et al. Consensus guidelines for microarray gene expression analyses in leukemia from three European leukemia networks. Leukemia 2006; 20: 1385–1392.
Erbilgin Y, Sayitoglu M, Hatirnaz O, Dogru O, Akcay A, Tuysuz G et al. Prognostic significance of NOTCH1 and FBXW7 mutations in pediatric T-ALL. Dis Markers 2010; 28: 353–360.
Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia 1995; 9: 1783–1786.
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003; 4: 249–264.
Simon R, Lam A, Li MC, Ngan M, Menenzes S, Zhao Y . Analysis of gene expression data using BRB-array tools. Cancer Inform 2007; 3: 11–17.
de Ridder D, Staal FJ, van Dongen JJ, Reinders MJ . Maximum significance clustering of oligonucleotide microarrays. Bioinformatics 2006; 22: 326–331.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3, RESEARCH0034.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 2006; 3: 995–1000.
Naishiro Y, Yamada T, Takaoka AS, Hayashi R, Hasegawa F, Imai K et al. Restoration of epithelial cell polarity in a colorectal cancer cell line by suppression of beta-catenin/T-cell factor 4-mediated gene transactivation. Cancer Res 2001; 61: 2751–2758.
Kolligs FT, Hu G, Dang CV, Fearon ER . Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol 1999; 19: 5696–5706.
Staal FJ, Noort MvM, Strous GJ, Clevers HC . Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep 2002; 3: 63–68.
Tong JH, To KF, Ng EK, Lau JY, Lee TL, Lo KW et al. Somatic beta-catenin mutation in gastric carcinoma—an infrequent event that is not specific for microsatellite instability. Cancer Lett 2001; 163: 125–130.
Horii A, Nakatsuru S, Miyoshi Y, Ichii S, Nagase H, Ando H et al. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res 1992; 52: 6696–6698.
Yamada Y, Oyama T, Hirose Y, Hara A, Sugie S, Yoshida K et al. beta-Catenin mutation is selected during malignant transformation in colon carcinogenesis. Carcinogenesis 2003; 24: 91–97.
Giles RH, van Es JH, Clevers H . Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003; 1653: 1–24.
Asally M, Yoneda Y . Beta-catenin can act as a nuclear import receptor for its partner transcription factor, lymphocyte enhancer factor-1 (lef-1). Exp Cell Res 2005; 308: 357–363.
Hovanes K, Li TW, Waterman ML . The human LEF-1 gene contains a promoter preferentially active in lymphocytes and encodes multiple isoforms derived from alternative splicing. Nucleic Acids Res 2000; 28: 1994–2003.
Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC . Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol 1998; 161: 3984–3991.
Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C . Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol 2006; 7: 1048–1056.
Mao TL, Chu JS, Jeng YM, Lai PL, Hsu HC . Expression of mutant nuclear beta-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J Pathol 2001; 193: 95–101.
Saegusa M, Hashimura M, Yoshida T, Okayasu I . beta-Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer 2001; 84: 209–217.
Kuhnl A, Gokbuget N, Kaiser M, Schlee C, Stroux A, Burmeister T et al. Overexpression of LEF1 predicts unfavorable outcome in adult patients with B-precursor acute lymphoblastic leukemia. Blood 118: 6362–6367.
Metzeler KH, Heilmeier B, Edmaier KE, Rawat VP, Dufour A, Dohner K et al. High expression of lymphoid enhancer-binding factor-1 (LEF1) is a novel favorable prognostic factor in cytogenetically normal acute myeloid leukemia. Blood 120: 2118–2126.
Gutierrez A, Sanda T, Ma W, Zhang J, Grebliunaite R, Dahlberg S et al. Inactivation of LEF1 in T-cell acute lymphoblastic leukemia. Blood 2010; 115: 2845–2851.
Klein EA, Assoian RK . Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci 2008; 121 (Pt 23): 3853–3857.
Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S et al. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem 2002; 277: 21657–21665.
Sayitoglu M, Erbilgin Y, Ng Hatirnaz O, Yildiz I, Celkan T, Anak S et al. Upregulation of T-cell specific transcription factor expression in pediatric T-ALL. Turk J Hematol 2012; 29: 325–333.
Benhaj K, Akcali KC, Ozturk M . Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep 2006; 15: 701–707.
Acknowledgements
This work is supported by the Research Fund of the Istanbul University (Project no. 355/03062005, 5785 and 11021), Turkish Society of Hematology, Scientific and Technical Research Council of Turkey-TUBITAK (Project no: 106S112 and 109S395), Turkish Republic Prime Ministry State Planning Organization-DPT (Project no: 2005K120430). O. Hatirnaz Ng was partially supported by EMBO short-term fellowship program (ASTF no: 326-2008) and Erasmus MC-Querido Chair Exchange Scholarship. FJT Staal is supported in part by the AICR (07-0049) and Kika (09-36).
Author contributions
OH Ng and M Sayitoglu designed the research and analyzed data; OH Ng performed microarray experiments, data analysis and TOP/FOP assays; Y Erbilgin performed mutation and protein analysis; S Fırtına performed QRT-PCR analysis; M Sayitoglu, OH Ng, Y Erbilgin and FJT Staal wrote the paper; JJM van Dongen and U Ozbek reviewed the manuscript; T Celkan, Z Karakas, G Aydogan, E Turkkan, Y Yıldırmak, CTimur and E Zengin provided the patient material and clinical data for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Ng, O., Erbilgin, Y., Firtina, S. et al. Deregulated WNT signaling in childhood T-cell acute lymphoblastic leukemia. Blood Cancer Journal 4, e192 (2014). https://doi.org/10.1038/bcj.2014.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2014.12
This article is cited by
-
FAT1 expression in T-cell acute lymphoblastic leukemia (T-ALL) modulates proliferation and WNT signaling
Scientific Reports (2023)
-
TCF-1: a maverick in T cell development and function
Nature Immunology (2022)
-
Cross-talk between GLI transcription factors and FOXC1 promotes T-cell acute lymphoblastic leukemia dissemination
Leukemia (2021)
-
Wnt signaling mediates oncogenic synergy between Akt and Dlx5 in T-cell lymphomagenesis by enhancing cholesterol synthesis
Scientific Reports (2020)
-
Wnt Signaling: Role in Regulation of Haematopoiesis
Indian Journal of Hematology and Blood Transfusion (2016)