Abstract
Chronic myelogenous leukemia (CML) evolves from a chronic phase characterized by the Philadelphia chromosome as the sole genetic abnormality and the accumulation of mature cells in peripheral blood into blast crisis, which is characterized by the rapid expansion of myeloid- or lymphoid-differentiation-arrested blast cells. Although ample studies have been conducted on the disease progress mechanisms, the underlying molecular mechanisms of the malignant phenotype transition are still unclear. In this study, we have shown that myofibrillogenesis regulator-1 (MR-1) was overexpressed in blast crisis patients and leukemic cells, but there was little trace expressed in healthy individuals and in most patients in CML chronic phase. MR-1 could inhibit the differentiation of myeloid cells into megakaryocytic lineages and accelerate cell proliferation. The molecular mechanism responsible for these effects was the interaction of MR-1 with MEK, which blocked the MEK/ERK signaling pathway by dephosphorylating MEK. Our results provide compelling and important evidence that MR-1 might act as a diagnostic marker and potential target of CML progression from chronic phase to blast crisis.
Similar content being viewed by others
Introduction
Chronic myelogenous leukemia (CML), a clonal myeloproliferative disorder of hematopoietic stem cells, is initially typically seen in a relatively benign chronic state, but finally turns into fatal blast crisis.1 In the chronic stage, the disease is indolent and the leukemic cells retain an ability to differentiate into mature granulocytes. After several years’ duration of the chronic phase, however, the disease inevitably accelerates and ultimately progresses to the terminal fatal stage, blast crisis, which is characterized by a large number of aggressive and immature blast cells.1, 2 The main obvious functional changes that occur with the progression of CML are the severe block in differentiation, apoptosis and acceleration in proliferation. These abnormalities of function and phenotype into blast are often associated with additional secondary chromosomal and molecular changes. Thus, the important and essential issues plaguing CML therapy are determination of the gene changes that cause disease progression, determination of the normal pathways that are subverted on the road to blast crisis and whether we can restore these pathways to put patients back into chronic phase.
Myofibrillogenesis regulator-1 (MR-1) was identified from a human skeletal muscle cDNA library (GenBank accession number AF417001) and is located on human chromosome 2q35 (GenBank accession number AC021016).3 MR-1 was seen to be overexpressed in human cancer cells.4 The ability of cell proliferation was decreased by MR-1 knockdown in human hepatoma cells and ovarian cancer cells.4, 5 Our previous results also revealed that MR-1 was almost undetectable in normal human lung fibroblasts and spleen cells but was overexpressed in human embryonic lung fibroblast cells and in some acute leukemic cell lines. It suggests that expression of MR-1 in normal tissue is low but is higher in its corresponding malignant or original tissue, hinting that MR-1 may be a proliferation- and differentiation-related gene and may participate in leukemic initiation and development.
CML blast crisis cells such as K562, MEG-01 and LAMA-84, derived from CML blast crisis patients,6, 7, 8 whose differentiation was severely impaired and biological behavior resembled acute leukemia, can be induced to differentiate into either lineage by exposure to various chemical agents.8, 9, 10 After phorbol-12-myristate (PMA) treatment, these cell lines were induced to differentiate forward megakaryocytic (MK) lineage and acquired the MK cellular and molecular features.11 The observed phenotypic transitions include cell-size increment, endomitosis, growth arrest, cell cycle arrest and expression of specific cell-surface markers such as CD41 and CD61.12, 13, 14 PMA-induced signaling cascade requires the MEK/ERK signaling activation to arrest the cell cycle and lead to cell-surface marker expression.13
In the present study, we verified that MR-1 was overexpressed in blast crisis patients and cell lines of CML but was not expressed or there was little trace of it in healthy individuals and in most patients in the chronic phase of CML. The knockdown of MR-1 inhibited proliferation and induced blast cell differentiation forward MK lineage through activation of the MEK/ERK signaling pathway induced by the upregulation of MEK phosphorylation.
Materials and methods
Cell culture and reagents
Blast crisis cell lines K562, MEG-01 and LAMA-84 were grown in RPMI-1640 medium supplemented with 10% fetal calf serum. K562 and LAMA-84 cells were kept in our laboratory, and MEG-01 was kindly provided by MS DeKui Mao (Military Medical Science Academy of the PLA). Human leukocytes were a generous gift from Dr Yajun Lin. MEK1/2 inhibitor U0126 and RIPA lysis were purchased from Cell Signaling Technology (Danvers, MA, USA). RAF inhibitor L779450 was purchased from Merck (Frankfurter, Darmstadt, Germany). Hoechst 33258 and Protein A+G agarose were from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China).
Patients
All the 13 patients (BCR-ABL positive) were from Beijing Daopei Hospital (10 patients) and the People’s Hospital of Beijing (three patients). Nine patients were in chronic phase and four patients were in blast crisis. All samples were obtained under the auspices of protocols approved by the Institutional Review Board, and total white cells were separated from the blood and bone marrow samples for western blot analysis. The definitions of chronic phase of CML and blast crisis of CML were based on the criteria of Sokal et al.15 (low risk: Sokal index <0.8; high risk: Sokal index >1.2) and the International Bone Marrow Transplant Registry. Thus, CP was defined as <10% blasts and BC as >30% blasts. The characteristics of the patients mentioned above are shown in Table 1.
siRNA preparation and treatment
21-nucleotide small interfering RNAs (siRNAs) were synthesized by Ribo Technology Company (Guangzhou, China) using 2′-ACE protection chemistry. Two siRNA sequences targeting MR-1 were 5′-ACCGUGUGAAGCAGAUGAAdTdT-3′ and 5′-CCUAGGCUAUUGACUGUUAdTdT-3′. Cells were collected and seeded in a six-well plate with 20 × 104 cells, and transfected with siRNA according to the manufacturer’s instructions.
Western blot
Whole-cell lysates were used for immunoblotting, as described previously.16 The anti-MR-1 polyclonal antibody was a generous gift from Professor Yiguang Wang (Institute of Medicinal Biotechnology, Beijing, China). Anti-phospho-Raf-1 (Ser338), anti-phospho-MEK1/2 (Ser217/221), anti-phospho-p44/42MAPK (ERK1/2), anti-phospho-p90RSK (Ser380), anti-phospho-MSK (mitogen-stress-activated protein kinase) (Thr581), anti-retinoblastoma protein (pRb), anti-caspase 3 and anti-p21 antibodies were all from Cell Signaling Technology. The anti-β-actin antibody was from Santa Cruz Biotechnologies (Santa Cruz, CA, USA), and horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibody was from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Electrochemiluminescence was performed according to the manufacturer’s instructions with a ChemiImager 5500 imaging system (Alpha Innotech, San Leandro, CA, USA).
Cell proliferation assay
For cell proliferation assay, K562 or other cells were seeded in a 24-well plate and the cells were counted by trypan blue-staining exclusion test. The cells were incubated with 0.4% (w/v) trypan blue for 5 min and analyzed by microscopy using a Neubauer hemocytometer. Only the cells that were not clearly stained blue were considered viable and the growth curve was depicted.
Construction of siRNA-expression plasmid
The DNA sequence against MR-1 was 5′-ACCGTGTGAAGCAGATGAATTCAAGAGATTCATCTGCTTCACACGGT-3′. pCD-shRNA (short hairpin RNA) was reconstructed from pcDNA3.0 (Invitrogen, Carlsbad, CA, USA). The corresponding DNA sequences were ligated into pCD-shRNA to form plasmids of pCD-shRNA-MR-1 and pCD-shRNA-mock.
Stable silencing of MR-1 in K562 cells
K562 cells at 60–70% confluence were transfected with 1 μg of pCD-shRNA-MR-1 or pCD-shRNA-mock using 1 μl lipofectamine LTX Reagent (Invitrogen) for 2 days. The transfected cells were exposed to 600 μg/ml G418 (Invitrogen) for 10 days, and then viable cells against G418 were collected and seeded at a lower density until a single colony was formed.
Flow cytometry analysis
To determine MK differentiation, 50 × 104 cells were incubated at 4 °C for 30 min in phosphate buffered saline (PBS) plus 1% fetal bovine serum containing anti-CD41-FITC monoclonal antibody (eBioscience, San Diego, CA, USA). Cells were analyzed by flow cytometry with a FACS Calibur (Coulter Epics XL-MCL, Becton Dickinson, Fullerton, CA, USA) using CellQuest software (Expo32 ADC).
For cell cycle and polyploidy analysis, cells were harvested and fixed overnight at 4 °C, as described previously.16 Thereafter, the cells were washed and resuspended in PBS containing 200 μg/ml RNase A at 37 °C for 30 min; this was followed by the addition of 50 μg/ml PI and the suspension was kept for 30 min in the dark. Cell analysis was performed using a FACS Calibur System.
Immunoprecipitation and dephosphorylation assay
For immunoprecipitation, cells were lysed with RIPA lysis (CST, Danvers, MA, USA). Cell lysates (500 μg of total protein for each sample) were incubated with 3 μg of specific antibodies overnight with constant rotation at 4 °C. Forty microliters of Protein A/G-Agarose were added, and the mixture was incubated for 3 h at 4 °Cwith gentle rocking; the mixture was then washed and subjected to SDS-PAGE and immunoblotting. A dephosphorylation assay was performed as described in (ref. 17). Protein containing p-MEK from K562/MR− cells was lysed and an equal amount of protein was loaded; western blot was performed using an anti-p-MEK antibody. Then, the membrane was stripped and each lane containing p-MEK was cut off and incubated with IP beads from Mock/K562 and K562/MR-1− cells or with blank lysate with MR-1 antibody in 120 mM Hepes, 12 mM DTT, 120 mm pNPP and 120 mM MgCl2 buffer at 30 °C for 30 min. Membranes were washed five times with TBS and bands were visualized with the p-MEK antibody, as described above.
Tumorigenicity assay
NOD/SCID mice aged 6–8 weeks from Chinese Academy of Medical Sciences (Beijing, China) were given commercial food, water ad libitum and housed at 23±5 °C and 55±5% RH throughout the experiment. 1700 × 106 K562/Mock and K562/MR-1−(2) cells were injected subcutaneously into the dorsal right flanks of recipient mice (n=5) in 0.2 ml PBS. Bidimensional tumor measurements were recorded every 3 days, and the average of these measurements was used to calculate the tumor volume. Mice were killed when tumor volume from the K562/Mock group reached 2000 mm3; the tumor loads were then dissected and weighed.
Statistical analysis
Data are expressed as arithmetic mean±s.d. Statistical analysis was performed using Student’s t-test. P<0.05 was considered statistically significant.
Results
MR-1 is overexpressed in blast crisis cells and downregulated in PMA-treated cells
Our results revealed that MR-1 was overexpressed in some Ph+ (K562) and non-Ph+ human acute leukemic cell lines (e.g., HL60, THP-1)18, 19 and was especially higher in CML blast crisis cells K562 as compared with the total white cells from healthy individuals, suggesting that MR-1 might have a more close relationship with blast crisis. To investigate the association between MR-1 overexpression and blast crisis, we further examined the MR-1 levels in four blast crisis patients, nine chronic-phase patients and nine healthy individuals and in blast crisis cell lines LAMA-84 and MEG-01 by western blot. As shown in Figure 1a, MR-1 was almost undetectable in total white cells from healthy individuals and in most patients in chronic phase of CML (however, one patient with low expression was seen). However, MR-1 was overexpressed in blast crisis patients and in blast crisis cell lines. These data indicated that MR-1 overexpression might be involved in the blast crisis transition characterized by differentiation arrest, the most typical event that occurs during progress.
MR-1 is overexpressed in leukemic and CML blast crisis cells and downregulated in PMA-treated cells. (a) Western blot analysis of MR-1 protein levels in the indicated cell lines from healthy individuals and CML patients. (b) Various cells were treated with the indicated concentrations of PMA for the indicated times and detected by western blot. The data are representative of three experiments and the patient’s information is described in Table 1.
PMA, an MK differentiation inducer, was introduced into our assay to show the association between MR-1 and differentiation. MR-1 levels decreased at 4 h of treatment in K562, LAMA-84 and MEG-01 cells, gradually declining further with longer treatment duration. At 48 h only trace MR-1 could be observed (Figure 1b).
MR-1 silencing inhibits cell proliferation and promotes MK differentiation
To investigate the function of MR-1, two siRNAs against MR-1 (siMR-1) were designed and synthesized to avoid the off-target effects, including siMR-1(1) and siMR-1(2). Both of these siRNAs could markedly reduce MR-1 levels and inhibit cell proliferation in 48-h-transfected K562 cells (Figure 2a) and had similar knockdown effect. However, the efficacy of siMR-1(1) was slightly better than that of siMR-1(2). Thus, MR-1-siRNA1 sequencing was chosen for the following assay.
Knockdown of MR-1 decreases cell proliferation and promotes MK differentiation in various leukemic cells. (a) After cells were transfected with various siRNAs (100 nM) for 48 h, MR-1 expression was analyzed by western blot (control MR/actin ratio was arbitrarily set to 1) and cell viability was monitored by trypan blue staining. (b) Cells were transfected with siRNAs (100 nM) twice within a 24-hour interval and reseeded for the indicated times to monitor cell viability; the initiatory cell number of Mock-siRNA (Mock) group was arbitrarily set to 1. (c) Cells were transfected with siRNAs (100 nM) twice within a 24-hour interval and then treated with 100 or 10 nM PMA in LAMA-84 or MEG-01 cells for 48 h and with 20 nM PMA in K562 cells for 96 h. CD41 expression was analyzed by flow cytometry. Data represent the mean value of three experiments, **P<0.01.
Similarly, MR-1 was markedly knocked down by siMR-1(1) in blast crisis cells LAMA-84 and MEG-01 (Figure 2a). Growth curve results showed that the proliferation of blast crisis cells was inhibited by 50–70% after two transfections at 24-h interval in K562, LAMA-84 and MEG-01 cells; especially in LAMA-84 and MEG-01 cells, the growth was almost arrested (Figure 2b). Flow cytometry results showed that the expression of MK differentiation marker CD41 was elevated after MR-1 silencing and treatment with PMA (Figure 2c).
Long-time MR-1 silencing triggers blast cell differentiation into cells with MK features
Differentiation is a phenotype-transforming event in which a number of proteins are synthesized to complete the whole cell, including cytoplasm, cell membrane and cell nucleus matter and this protein synthesis needs a stable, long-term internal environment. Therefore, we established MR-1 stable silencing cells K562/MR-1−(1) and K562/MR-1−(2) to observe the effects of long-term-silencing MR-1 on blast cell differentiation. In K562/MR-1− cells, the expression of MR-1 was markedly decreased in the range of 40–70%, compared with K562/Mock cells (Figure 3a). The MR-1 silencing efficacy of K562/MR-1−(2) cells was more obviously and mostly used for the following experiments.
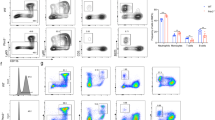
Long-term MR-1 silencing triggers the differentiation of blast cells into cells with MK cellular features. (a) Western blot analysis of MR-1 protein levels in various MR-1 stable silencing K562/MR-1− cells. (b) Growth curves of K562/MR-1−(1), K562/MR-1−(2) and K562/Mock cells. (c) CD41 expression and (d) cell cycle distribution were analyzed by flow cytometry. (e) Western blot analysis of p21, pRb and caspase 3. (f) Light microscopic analysis and (g) Wright–Giemsa staining analysis of megakaryocytic features. (h) The morphological aspects of the polyploid nature of K562/MR-1− cells were measured by Hoechst 33258 staining and light microscopy. (i) The diploid of K562/MR-1− and K562/Mock cells was detected by flow cytometry. (j) The morphology of apoptosis of K562/MR-1− cells was visualized by Hoechst 33258 staining and light microscopy. (k) Apoptosis analysis of K562/MR-1− cells by flow cytometry. Data represent mean values of three experiments, *P<0.05.
The growth curve showed that cell growth in MR-1-silencing cells was significantly less than that in K562/Mock cells, especially in K562/MR-1−(2) cells (Figure 3b). Moreover, the FACS analysis showed that the fluorescence intensity of CD41 was 4.8, 6.7 and 0.3% for K562/MR-1−(1), K562/MR-1−(2) and K562/Mock cells, respectively (Figure 3c). These data indicated that the expression of CD41 was higher in MR-1-silencing cells than in K562/Mock cells.
Cell cycle analysis showed that the G0/G1 accumulation in K562/MR-1−(1) and K562/MR-1−(2) cells was ∼50–60% as compared with K562/Mock cells (Figure 3d), demonstrating a G0/G1 arrest in MR-1-silencing cells. Simultaneously, the expression of p21 (cyclin-dependent kinase inhibitor) was significantly increased, and the expression of pRb and caspase 3 was reduced in MR-1-silencing cells (Figure 3e). Particularly in K562/MR-1−(2) cells, the degrees of MR-1 knockdown, proliferation inhibition and differentiation ability were stronger; the degree of G0/G1-phase arrest was also more obvious as compared with that in K562/MR-1−(1) cells. Moreover, the degree of cell cycle arrest coincided with the change in degree of p21 and pRb levels (Figure 3e).
Some MK morphological characters were also observed in MR-1-silencing cells. A net increase in cell size was visualized in MR-1-silencing K562/MR-1−(1) and K562/MR-1−(2) cells, in which a fraction of large-sized cells whose diameter was 40–60 μm as well as some huge vacuoles from the larger sized cells were observed. A cell bulge that seemed like a cell budding was also visualized in some large cells. At late stage, the large cells lysed and formed cell debris (Figure 3f i-v). The other MK features such as the increase in nuclear size and nuclear–cytoplasmic ratio were also observed in K562/MR-1−(2) cells by Wright–Giemsa staining (Figure 3g). Polyploidy cells were observed in K562/MR-1−(2) cells, including 8N, 16N and 32N cells by Hochest 33258 stain analysis (Figure 3h). The corresponding quality analysis showed that the number of polyploidy cells of MR-1-silencing cells was 2–3-fold of K562/Mock cells as assessed by FACS, and the ratio of polyploidy cells in K562/MR-1−(1), K562/MR-1−(2) and K562/Mock cells was 4.1, 5.3 and 1.5%, respectively (Figure 3i). Apoptotic DNA fragments of large-sized cells were also observed in K562/MR-1−(2) cells (Figure 3g). The ratio of apoptotic cells in K562/MR-1−(1), K562/MR-1−(2) and K562/Mock cells was 4.2, 9.1 and 2.4%, respectively (Figure 3k).
Enhancement of PMA-induced MK differentiation by MR-1 silencing
To further study the role of MR-1 in MK differentiation, we investigated whether MR-1 silencing could promote MK differentiation induced by PMA. After 48 h of PMA treatment, clusters of a large number of cells were increased in MR-1-silencing cells, and some vacuole cells were observed in K562/MR-1−(2) cells (Figure 4a). After 96-h treatment, the vacuole cells were increased in MR-1-silencing cells, and the size of these cells was larger than that of K562/Mock cells (Figure 4b). Moreover, we found that the CD41 expression in MR-1-silencing cells was higher than that in K562/Mock cells after 96-h treatment (Figure 4c), and the fluorescence intensity of CD41 was 83.9, 72.9 and 41.5% for K562/MR-1−(2), K562/MR-1−(1) and K562/Mock cells, respectively, consistent with the morphological appearance of MK differentiation of K562/MR-1−(2) and K562/MR-1−(1) cells treated by PMA. Further, the proliferative ability of MR-1-silencing cells was further decreased as compared with K562/Mock cells (Figure 4d).
MR-1 silencing promoted MK differentiation induced by PMA. K562/MR-1− cells were treated with 20 nM PMA for 48 h (a) or for 96 h (b), and then the megakaryocytic features were analyzed by light microscopy. After 96-h treatment, CD41 expression (c) and cell viability (d) were measured by FACS and cell count assay, respectively. *P<0.05; **P<0.01.
Involvement of MEK/ERK signal in MK differentiation by MR-1-mediated MEK dephosphorylation
Although p38, JUK and MEK/ERK signaling cascades participated in the differentiation of CML,20 only the MEK/ERK signal pathway has an essential role in MK differentiation.21 We further investigated the relationship between MEK/ERK signaling cascades and MR-1-silencing-induced MK differentiation. The results showed that the phosphorylation forms of MEK1/2 and ERK1/2 were markedly increased in MR-1-silencing K562 cells. Further, the downstream proteins ribosomal S6 kinase 90 and MSK also acquired more phosphorylated forms (Figure 5a). The phosphorylation levels of signal proteins in K562/MR-1−(2) cells were higher than that in K562/MR-1−(1), in line with the higher MK differentiation level compared with K562/MR-1−(1). Moreover, cyclin D1 expression, which is attributed to polyploidy formation during MK differentiation, was upregulated in MR-1-silencing cells (Figure 5a).
The MEK/ERK signal involved in MK differentiation by MR-1-mediated MEK dephosphorylation. Western blot analysis of various signal proteins. (a) Cells were treated with 20 μM U0126 for 2 h or with 10 μM L779450 for 1 h. Various signal proteins were detected by WB (up panel), and CD41 was analyzed by FACS; cell viability was monitored by cell count (b). Interaction of MR-1 and MEK was analyzed by reciprocal immunoprecipitation (c). Triplicate cell lysates from K562/Mock were subjected to western blot for p-MEK detection, then stripped and cut into thirds, each lane containing an equal amount of p-MEK incubated with IP MR-1 from blank control (lane 1), Mock/K562 (lane 2) or K562/MR-1− cell lysate (lane 3) for 30 min, followed by p-MEK western blot analysis. (d) The data are the means±s.d. of two experiments; significance of Student’s t-test: *P<0.05.
To confirm whether MK differentiation was induced through the MEK/ERK pathway in MR-1-silencing cells, the MEK inhibitor U012622, 23 was used. As shown in Figure 5b, ERK phosphorylation was reversed by U0126 in K562/MR-1−(2) cells. U0126 also reversed the expression of CD41 and the growth inhibition of K562/MR-1−(2) cells.
Our data showed that RAF activation was not observed in MR-1-silencing cells, hinting that MEK activation was not regulated by RAF. To further confirm that MEK activation was independent of RAF, RAF inhibitor L77945024, 25 was used. The results showed that L779450 did not affect the phosphorylation of MEK and ERK in MR-1-silencing cells, although it inhibited MEK and ERK phosphorylations in Mock cells (Figure 5b). On account of the MEK phosphorylation increase being independent of RAF in MR-1-silencing cells, we assumed that MR-1 might directly interact with MEK and dephosphorylate MEK to inhibit MEK activation. Therefore, reciprocal coimmunoprecipitation (IP) was performed and the results showed that there was an interaction between MR-1 and MEK (Figure 5c). In vitro dephosphorylation assay further showed that p-MEK incubated with IP MR-1 protein from K562/Mock cells (Figure 5d, lane 3) was significantly lower than that from MR-1-silencing cells (Figure 5d, lane 2) or blank control (Figure 5d, lane 1), indicating that MR-1 could significantly dephosphorylate p-MEK.
In vivo reduction of K562/MR-1 tumorigenicity
To further validate that MR-1 silencing can reduce K562 cell proliferation, K562/Mock and K562/MR-1− cells were injected subcutaneously into the dorsal right flanks of NOD/SCID mice, and tumor volumes were measured every 3 days (Figure 6a). Seven days after tumor inoculation, a tiny tumor nodule was seen only in the K562/Mock group, and the tumor grew quickly. However, the tumorigenicity of the K562/MR-1− group was markedly limited (Figure 6a). After 31 days, the mice were killed, and the tumor loads were resected and assessed (Figure 6b). The average weight of K562/Mock and K562/MR-1− tumors was 2.5 and 0.4 g, respectively (Figure 6b), validating a pivotal role of MR-1 in tumorigenicity.
Disussion
CML treatment depends on the phase of the disease, age of the patients and their general health. During the chronic phase, the aim of treatment is to control the blood counts to within a normal range and perform a bone marrow transplantation.2 In the blast phase, as the number of blast cells markedly increases in the blood and bone as a result of additional chromosomal and molecular changes during the disease progress, the aim of the treatment during the advanced stages is to destroy the leukemic cells and allow the bone marrow to function normally again, or to return the patient to the chronic phase of their disease.26 Thus, it is essential to clarify the additional gene changes that promote the differentiation arrest in the crisis transition to convert the patients back into chronic phase. The previous data showed that MR-1 was overexpressed in cancer cells and promoted cell proliferation and metastasis.4 In this study, we found that MR-1 was overexpressed in Ph+ or non-Ph+ leukemic cells and in whole white cells from blast crisis patients and in weakly differentiated blast crisis cells, but was not expressed or there was little trace in whole white cells from healthy individuals and in most patients in blast crisis of CML, suggesting that MR-1 might be involved in the malignant phenotype transition. Further, MR-1 was markedly decreased by the treatment of PMA, an MK differentiation inducer. Notably, the downregulation of MR-1 was accompanied by the MK differentiation induced by PMA, indicating that MR-1 might have an inhibitory role in MK differentiation.
We hypothesized that MR-1 was involved in MK differentiation as a negative regulator. First, the knockdown of MR-1 decreased the proliferation of blast crisis cells and increased CD41 expression (MK differentiation biomarker). Second, the stable knockdown of MR-1 showed that whole cells displayed MK differentiation characters, including cytoplasm enlargement, endomitosis, cell cycle arrest, CD41 increase and cytoplasmic projection form.12, 27 These changes were the typical cellular features of MK differentiation.6 Further, these differentiated large-sized cells were also polyploid in nature and eventually underwent apoptosis, indicating that MR-1 silencing could promote the development of some blast cells into mature MK cells.28
Next, we explored the molecular signaling pathway that linked MR-1 with MK differentiation and proliferation. It is well known that the activation of MEK/ERK is essential for the MK differentiation of K562 cells induced by PMA.12, 29 The expression of constitutively activated MEK or ERK induces the transition to MK lineage and many MK development hallmarks.19, 30, 31 In our study, MR-1 silencing promoted blast cell differentiation into MK lineage and activated the MEK/ERK pathway strongly. After the MEK kinase inhibitor U0126 blocked ERK phosphorylation, MK differentiation was inhibited, and proliferation inhibition was also reversed in MR-1-silencing cells, However, L779540, a RAF kinase inhibitor, could not markedly downregulate the phosphorylations of MEK and ERK in MR-1-silencing K562 cells, indicating that the upregulation of MK differentiation and the downregulation of cell proliferation induced by MR-1 silencing were dependent on the activation of MEK and ERK and independent of RAF. In vitro reciprocal IP and dephosphorylation assays further demonstrated that MR-1 interacted with MEK to dephosphorylate MEK. It is worthy to investigate its detailed mechanism in the future.
Sustained ERK activation promotes the upregulation of p21 and cyclin D132, 33, 34 and activates caspase 3, all of which have an important role in the late steps of physiological MK differentiation.35 In our study, p21 was upregulated and pRb was downregulated in MR-1-silencing cells, and these changes led to G0/G1 arrest. Usually, the increased G0/G1 phase is necessary for completing some important events of MK differentiation, including the development of cytoplasm and the expression of CD41 and some essential proteins.36 Among hematopoietic cells, differentiation to megakaryocytes is associated with repeated rounds of DNA synthesis without the occurrence of cell division, a process referred to as endomitosis.37 The upregulation of cyclin D1 and p21 contributes to endomitosis formation.38 Our results showed that the downregulation of MR-1 could induce upregulation of p21 and cyclin D1, indicating the involvement in polyploidy formation. Apart from contributing to polyploidy formation, cyclin D1 was beneficial for the increase in cell size and DNA content;38 these data accounted for these phenotype features of MK differentiation occurring in MR-1-silencing cells. Thus, the downregulation of MR-1 in K562 cells initiated events of spontaneous MK differentiation, such as expression of specific cell-surface markers, inhibition of cell proliferation and polyploidization.
Overall, MR-1 as a new regulator is involved in the formation of the cellular malignant phenotype of the blast transition and has a pivotal role in MK differentiation arrest. MR-1 silencing triggers MK differentiation and inhibits proliferation by sustained activation of the MEK/ERK pathway, which was a result of the downregulation of MEK phosphorylation mediated by MR-1. Our findings provide the compelling and important evidence that MR-1 might act as a diagnostic marker of CML progression from chronic phase to blast crisis.
References
Savage DG, Szydlo RM, Goldman JM . Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period. Br J Haematol 1997; 96: 111–116.
Calabretta B, Perrotti D . The biology of CML blast crisis. Blood 2004; 103: 4010–4022.
Li TB, Liu XH, Feng S, Hu Y, Yang WX, Han Y et al. Characterization of MR-1, a novel myofibrillogenesis regulator in human muscle. Acta Biochim Biophys Sin (Shanghai) 2004; 36: 412–418.
Ren K, Jin H, Bian C, He H, Liu X, Zhang S et al. MR-1 modulates proliferation and migration of human hepatoma HepG2 cells through myosin light chains-2 (MLC2)/focal adhesion kinase (FAK)/Akt signaling pathway. J Biol Chem 2008; 283: 35598–35605.
Lu R, Sun M, Feng J, Gao X, Guo L . Myofibrillogenesis regulator 1 (MR-1) is a novel biomarker and potential therapeutic target for human ovarian cancer. BMC Cancer 2011; 11: 270–279.
Lozzio BB, Lozzio CB . Properties of the K562 cell line derived from a patient with chronic myeloid leukemia. Int J Cancer 1976; 18: 421–431.
Ogura M, Morishima Y, Ohno R, Kato Y, Hirabayashi N, Nagura H et al. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood 1985; 66: 1384–1392.
Seigneurin D, Champelovier P, Mouchiroud G, Berthier R, Leroux D, Prenant M et al. Human chronic myeloid leukemic cell line with positive Philadelphia chromosome exhibits megakaryocytic and erythroid characteristics. Exp Hematol 1987; 15: 822–832.
Kawano T, Horiguchi-Yamada J, Iwase S, Furukawa Y, Kano Y, Yamada H . Inactivation of ERK accelerates erythroid differentiation of K562 cells induced by herbimycin A and STI571 while activation of MEK1 interferes with it. Mol Cell Biochem 2004; 258: 25–33.
Genever PG, Wilkinson DJ, Patton AJ, Peet NM, Hong Y, Mathur A et al. Expression of a functional N-methyl-D-aspartate-type glutamate receptor by bone marrow megakaryocytes. Blood 1999; 93: 2876–2883.
Levay K, Slepak VZ . Tescalcin is an essential factor in megakaryocytic differentiation associated with Ets family gene expression. J Clin Invest 2007; 117: 2672–2683.
Herrera R, Hubbell S, Decker S, Petruzzelli L . A role for the MEK/MAPK pathway in PMA-induced cell cycle arrest: modulation of megakaryocytic differentiation of K562 cells. Exp Cell Res 1998; 238: 407–414.
Long MW, Heffner CH, Williams JL, Peters C, Prochownik EV . Regulation of megakaryocyte phenotype in human erythroleukemia cells. J Clin Invest 1990; 85: 1072–1084.
Colamonici OR, Trepel JB, Neckers LM . Phorbol ester enhances deoxynucleoside incorporation while inhibiting proliferation of K-562 cells. Cytometry 1985; 6: 591–596.
Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE et al. Prognostic discrimination in ‘good-risk’ chronic granulocytic leukemia. Blood 1984; 63: 789–799.
Chen XL, Ren KH, He HW, Shao RG . Involvement of PI3K/AKT/GSK3beta pathway in tetrandrine-induced G1 arrest and apoptosis. Cancer Biol Ther 2008; 7: 1073–1078.
Ferrero GO, Velazquez FN, Caputto BL . The kinase c-Src and the phosphatase TC45 coordinately regulate c-Fos tyrosine phosphorylation and c-Fos phospholipid synthesis activation capacity. Oncogene 2012; 31: 3381–3391.
Evans JP, Wickremasinghe RG, Hoffbrand AV . Tyrosine protein kinase substrates in Philadelphia-positive human chronic granulocytic leukemia derived cell lines (K562 and BV173): detection by using an immunoblotting technique. Leukemia 1987; 1: 524–525.
Bandyopadhyay G, Biswas T, Roy KC, Mandal S, Mandal C, Pal BC et al. Chlorogenic acid inhibits Bcr-Abl tyrosine kinase and triggers p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemic cells. Blood 2004; 104: 2514–2522.
Severin S, Ghevaert C, Mazharian A . The mitogen-activated protein kinase signaling pathways: role in megakaryocyte differentiation. J Thromb Haemost 2010; 8: 17–26.
Melemed AS, Ryder JW, Vik TA . Activation of the mitogen-activated protein kinase pathway is involved in and sufficient for megakaryocytic differentiation of CMK cells. Blood 1997; 90: 3462–3470.
Miyazaki R, Ogata H, Kobayashi Y . Requirement of thrombopoietin-induced activation of ERK for megakaryocyte differentiation and of p38 for erythroid differentiation. Ann Hematol 2001; 80: 284–291.
Guerriero R, Mattia G, Testa U, Chelucci C, Macioce G, Casella I et al. Stromal cell-derived factor 1alpha increases polyploidization of megakaryocytes generated by human hematopoietic progenitor cells. Blood 2001; 97: 2587–2595.
Shelton JG, Moye PW, Steelman LS, Blalock WL, Lee JT, Franklin RA et al. Differential effects of kinase cascade inhibitors on neoplastic and cytokine-mediated cell proliferation. Leukemia 2003; 17: 1765–1782.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N . RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010; 464: 427–430.
Radich JP . The Biology of CML blast crisis. Hematology Am Soc Hematol Educ Program 2007, 384–391.
Schulze H, Korpal M, Hurov J, Kim SW, Zhang J, Cantley LC et al. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood 2006; 107: 3868–3875.
Mathur A, Hong Y, Wang G, Erusalimsky JD . Assays of megakaryocyte development: surface antigen expression, ploidy, and size. Methods Mol Biol 2004; 272: 309–322.
Chang YI, Hua WK, Yao CL, Hwang SM, Hung YC, Kuan CJ et al. Protein-arginine methyltransferase 1 suppresses megakaryocytic differentiation via modulation of the p38 MAPK pathway in K562 cells. J Biol Chem 2010; 285: 20595–20606.
Whalen AM, Galasinski SC, Shapiro PS, Nahreini TS, Ahn NG . Megakaryocytic differentiation induced by constitutive activation of mitogen-activated protein kinase kinase. Mol Cell Biol 1997; 17: 1947–1958.
Racke FK, Lewandowska K, Goueli S, Goldfarb AN . Sustained activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway is required for megakaryocytic differentiation of K562 cells. J Biol Chem 1997; 272: 23366–23370.
Balmanno K, Cook SJ . Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 1999; 18: 3085–3097.
Coqueret O . Linking cyclins to transcriptional control. Gene 2002; 299: 35–55.
Coqueret O . New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol 2003; 13: 65–70.
Colosetti P, Puissant A, Robert G, Luciano F, Jacquel A, Gounon P et al. Autophagy is an important event for megakaryocytic differentiation of the chronic myelogenous leukemia K562 cell line. Autophagy 2009; 5: 1092–1098.
Meshkini A, Yazdanparast R . Involvement of ERK/MAPK pathway in megakaryocytic differentiation of K562 cells induced by 3-hydrogenkwadaphnin. Toxicol In Vitro 2008; 22: 1503–1510.
Vitrat N, Cohen-Solal K, Pique C, Le Couedic JP, Norol F, Larsen AK et al. Endomitosis of human megakaryocytes are due to abortive mitosis. Blood 1998; 91: 3711–3723.
Wilhide CC, Van Dang C, Dipersio J, Kenedy AA, Bray PF . Overexpression of cyclin D1 in the Dami megakaryocytic cell line causes growth arrest. Blood 1995; 86: 294–304.
Acknowledgements
This work was supported by grants from NSFC (No. 30772583), the National 973 Program (No. 2009CB521807) and National S&T Major Special Project on Major New Drug Innovation (No. 2012ZX09301-002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Zhao, W., He, H., Ren, K. et al. MR-1 blocks the megakaryocytic differentiation and transition of CML from chronic phase to blast crisis through MEK dephosphorylation. Blood Cancer Journal 3, e107 (2013). https://doi.org/10.1038/bcj.2013.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2013.5
Keywords
This article is cited by
-
Establishment of a novel mesenchymal stem cell-based regimen for chronic myeloid leukemia differentiation therapy
Cell Death & Disease (2021)
-
The novel quinolizidine derivate IMB-HDC inhibits STAT5a phosphorylation at 694 and 780 and promotes DNA breakage and cell apoptosis via blocking STAT5a nuclear translocation
Acta Pharmacologica Sinica (2020)
-
Cyclizing-berberine A35 induces G2/M arrest and apoptosis by activating YAP phosphorylation (Ser127)
Journal of Experimental & Clinical Cancer Research (2018)
-
Genetic Events Other than BCR-ABL1
Current Hematologic Malignancy Reports (2014)